
Quantitative iTRAQ proteomics reveal the proteome profiles of bone marrow mesenchymal stem cells after cocultures with Schwann cells in vitro
Introduction
Spinal cord injury (SCI) has limited treatment options, and is characterized by high morbidity and disability (1). Currently, no effective treatment method is available for SCI (2). SCI can be divided into primary and secondary injuries based on pathology. A secondary injury comprises a series of complex reactions involving factors, such as free radicals, calcium-ion influx, and macrophage polarization, which can contribute to more severe damage (3-8). Later, glial scar formation and an imbalance in the microenvironment may also prevent the repair of the injured spinal cord. Various therapeutic methods, including cell graft therapy, have been applied to improve functional recovery after SCI. The administration of methylprednisolone, which was once the only Food and Drug Administration-approved drug for the treatment of traumatic acute SCI, has dramatically decreased in many regions, however, some clinicians still believe in its efficacy (9-13).
The transplantation of stem cells provides a potential method to replace injured cells at lesion sites for the repair of SCI, and bone marrow mesenchymal stem cells (BMSCs) are one of the most studied cell types (14-16). BMSCs facilitate the healing of ischemic tissue-related diseases through proangiogenic secretory proteins (17). BMSCs have several beneficial properties, including low immunogenicity, the secretion of a variety of growth factors, and pluripotency, which enable the formation of different phenotypes in response to changes in elasticity at the tissue level (18,19).
Schwann cells (SCs) play significant roles in peripheral nerve injury, a process that is related to different types of macrophages (20). Our previous research revealed that the transplantation of SCs, which is an effective therapeutic method, promotes axonal regeneration and functional recovery after SCI in rats, but the mechanism by which this occurs remains unclear (21-24). We also found that co-transplanting BMSCs with SCs better promotes functional recovery in rats after SCI (23). To explore the potential mechanism underlying the interaction between these two types of cells, we used isobaric tag for relative and absolute quantitation (iTRAQ) to detect differentially expressed proteins (DEPs) in BMSCs cocultured with SCs. iTRAQ analyses on SCI are mainly related to pathological mechanism. However, this study is a continuation of our previous studies just as mentioned. We aim to investigate the potential repair mechanism of co-transplantation BMSCs with SCs which is first reported. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3073/rc).
Methods
Animals
Adult female Wistar rats (Pasteur Institute, China) weighing 180–200 g (n=20) was used in this study. All the procedures in this study, including the use of animals, were approved by the Tianjin Medical University Ethical Committee (No. TMUAMEC2017025) and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publications, revised 2011). The animals were randomly used for the BMSC and SC cultures. A protocol was prepared without registration before the study.
BMSC and SC cultures
The methods used for the BMSC and SC primary cultures were performed as previously described (23,25) with some modifications. Briefly, the rats were anesthetized with 3% pentobarbital sodium (45 mg/kg intraperitoneally) and sacrificed by cervical dislocation. The bilateral sciatic nerve was exposed, and 20 mm of the distal segment of the nerve was resected and placed in a dish containing phosphate buffered solution (Sigma, Germany). After the connective tissue and epineurium were cautiously pulled away with fine forceps under sterile conditions, the remaining nerves were teased apart with a needle and cut into fragments of 2 to 3 mm. The disentangled nerve fragments were digested in a 15-mL sterilized tube containing 0.3% collagenase type II (Invitrogen, USA) at 37 ℃ for 30 minutes with agitation. After the collagenase was removed carefully, the sample was incubated with 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA; Gibco, USA) for 5 minutes in a 37 ℃ incubator. At the same time, both the bilateral femurs and tibias were removed under sterile conditions. The epiphyses were removed and the bone marrow was flushed with Dulbecco’s modified Eagle’s medium/nutrient mixture F12 (1:1 D/F12, Gibco, USA). The resulting bone marrow fluid was filtered through a 70-µm nylon mesh. Both the SCs and BMSCs were cultured in 75-cm2 flasks using D/F12 supplemented with 10% fetal bovine serum (Gibco, USA) and 100 U/mL of penicillin and streptomycin (Gibco, USA) at 37 ℃ in a 5% carbon dioxide incubator (ThermoFisher, USA). For the SCs, the basic medium was replaced with a purification medium [a basic medium containing 10 µM of cytosine β-D-arabinofuranoside (Sigma, Germany)] to eliminate the fibroblasts. The purification medium was changed to growth medium [a basic medium containing 20 ng/mL of heregulin 1-β1 (HRG1-β1) extracellular domain (ECD) (R&D systems, USA)] 24 hours later. The medium was changed and replaced every 2–3 days with fresh complete culture medium. The BMSCs and SCs were used for experiments at passages 3–5.
Coculture system for BMSCs and SCs
In the present study, a semi-quantitative medium exchange method was used to coculture the BMSCs and SCs as previously described (23). Briefly, the BMSC medium was completely replaced with 5 mL of BMSC medium and 5 mL of refresh medium. Both cocultures and control cultures were incubated for 3 and 7 days. The following three groups were created and had various coculture durations: Group 1: the BMSCs were cocultured with SCs for 7 days (the SC7d group); Group 2: the BMSCs were cocultured with SCs for 3 days (the SC3d group); and Group 3: the BMSCs were cultured alone (the SC0d group, which served as the control group). Additionally, three replicates with independent samples were used to ensure the reproducibility of the results.
iTRAQ sample preparation
The cell precipitates were homogenized on ice, and 300 mg of homogenate from each group was used for the proteomic screening (26). All the cell samples were lysed with a mixture of 8 M of urea, 50 mM of Tris (pH 8.0), 1% Nonidet P-40 (NP-40), 1% sodium deoxycholate, 1% protease inhibitor, 2 mM of EDTA and 10 mM of dithiothreitol (DTT). A two-dimensional Quant kit (GE Healthcare, USA) was used to establish the protein concentrations. After digestion overnight at 37 ℃ with trypsin (50 µg/mL), the protein samples (250 mg) were labeled with the iTRAQ reagents (5-plex; AB SCIEX, MA, USA) in accordance with the manufacturer’s protocol.
Data analysis and bioinformatics
Using the software Protect Discoverer version 1.2, the raw data files acquired from the Orbitrap were converted into a Mascot input file (MGF files) that contained secondary spectrum information. The MGF files were then imported into Mascot software version 2.3 for qualitative and quantitative calculations. The qualitative and quantitative protein information was exported to a comma-separated values file containing all the information for the subsequent analysis. To detect the biological and functional characteristics of all the DEPs, the Gene Ontology (GO) database was used to map the sequences. To identify candidate biomarkers in this process, a pathway analysis was conducted using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. The protein-protein interaction (PPI) network was analyzed using the Search Tool for the Retrieval of Interacting Genes (STRING) database.
Immunofluorescence staining
The cells were fixed on ice with 4% paraformaldehyde for 15 min. All the staining procedures were performed as previously described (23). Primary antibody S100 (Abcam, USA; ab52642) was diluted in 0.25% Triton X-100 at 1:200. Secondary antibody goat anti-rabbit Alexa Fluor 488 (Abcam, USA; ab150077) was diluted in 0.25% Triton X-100 at 1:500. Images were taken with a fluorescent microscope.
Multilineage differentiation of BMSCs
BMSCs at passage 3 were seed with a concentration of 2×105/mL on a 6-well plate. When the cells reached 100% confluence, changed the differentiation medium and incubated for 10 days. For adipogenic differentiation, the differentiation medium comprised the basic medium with 0.1 µM dexamethasone, 10 µg/mL insulin, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX). Oil red O stain solution was used to show adipocytes. All the regents were provided by the rat BMSC adipogenic differentiation kit (Chem, China; CHEM-200014). For chondrogenic differentiation, the differentiation medium comprised the basic medium with 0.1 µM dexamethasone, 50 µg/mL ascorbic acid, 6.25 µg/mL insulin, 6.25 µg/mL transferrin. Alcian blue cartilage stain solution was used to show chondrocytes. All the regents were provided by the rat BMSC chondrogenic differentiation kit (Chem, China; CHEM-200015). For osteogenic differentiation, the differentiation medium comprised the basic medium with 1 µM dexamethasone, 50 µg/mL ascorbic acid, 10 mM sodium β-glycerophosphate. Alizarin red stain solution was used to show osteoblasts. All the regents were provided by the rat BMSC osteogenic differentiation kit (Chem, China; CHEM-200016).
Western blot
Western blot was performed as previously described with a minor modification (27). After the cocultures, the BMSCs were lysed with radioimmunoprecipitation supplemented with phenylmethylsulfonyl fluoride. The protein samples were electrophoresed in 10% sodium dodecyl sulfate gel and transferred to polyvinylidene fluoride membranes at 4 ℃. The membranes were cut according to the molecular weight and blocked in 5% skim milk at room temperature for 1 h. Primary antibodies, including anti-collagen VI alpha 2 (Col6a2), anti-intercellular adhesion molecule-1 (ICAM1), anti-Grb2, anti-Col4a2, and anti-P4hb, were used to probe the membranes at 4 ℃ overnight. The membranes were then incubated with secondary antibodies at room temperature for 1 h. The bands were visualized by chemiluminescence.
Statistical analysis
The statistical analysis was performed using SPSS version 15. A one-way analysis of variance with a post-hoc Newman-Keuls multiple comparison test was conducted to identify significant differences among the groups. A P value <0.05 was considered statistically significant.
Results
Cell identification
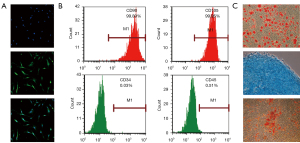
As Figure 1A shows, the SCs were positive for S100. The flow cytometry revealed that the BMSCs were positive for the well-defined BMSC markers of cluster differentiation (CD)90 and CD105, but negative for the hematopoietic surface antigens of CD34 and CD45 (see Figure 1B). Further, the BMSCs showed the ability to differentiate into adipocytes, chondrocytes and osteoblasts (see Figure 1C). These results indicate that the primary cultures of SCs and BMSCs were successful and could be used for the subsequent proteomics analysis.
DEPs identified by proteomic analysis
In total, 6,760 proteins were identified, of which 5,184 were quantified. Trends in changes in protein expression were investigated for the BMSC and SC cocultures for the SC3d and SC7d groups relative to the SC0d group. The differences and similarities in protein differential expression were analyzed. The DEPs were regarded when the difference magnitude among the groups was >1.3-fold, and the result was reproduced twice.
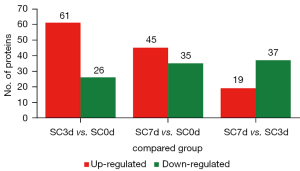
The number of DEPs is shown in Figure 2. After comparing the BMSCs cocultured with the SCs for 3 days to the control group (SC3d vs. SC0d), 87 DEPs were identified, of which 61 were upregulated and 26 were downregulated (see Table S1). After comparing the BMSCs cocultured with the SCs for 7 days to the control group (SC7d vs. SC0d), 80 DEPs were identified, of which 45 were upregulated and 35 were downregulated (see Table S2). After comparing the BMSCs cocultured with the SCs for 7 days to those cocultured for 3 days (SC7d vs. SC3d), 56 DEPs were identified, of which 19 were upregulated and 37 were downregulated (see Table S3).
GO analysis
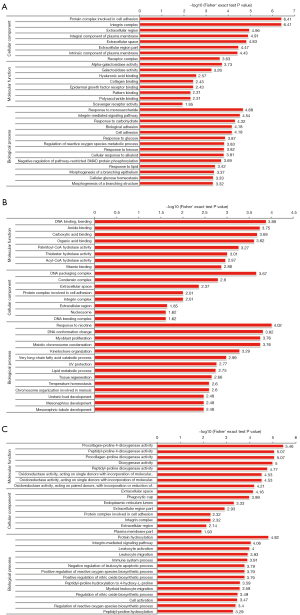
In the GO analysis, the genes or proteins were assessed based on the following three features: biological processes, molecular functions, and cellular components. In relation to the 87 DEPs in the SC3d vs. SC0d comparison, the top 10 significantly enriched GO terms were “protein complex involved in cell adhesion”, “integrin complex”, “extracellular region”, “integral component of plasma membrane”, “extracellular space”, “response to monosaccharide”, “integrin-mediated signaling pathway”, “extracellular region part”, “intrinsic component of plasma membrane”, and “response to carbohydrate” (see Figure 3A). In relation to the 80 DEPs in the SC7d vs. SC0d comparison, the top 10 significantly enriched GO terms were “response to nicotine”, “DNA binding, bending”, “DNA conformation change”, “myoblast proliferation”, “meiotic chromosome condensation”, “amide binding”, “carboxylic acid binding”, “DNA packaging complex”, “organic acid binding”, and “kinetochore organization” (see Figure 3B). Finally, in relation to the 56 DEPs in the SC7d vs. SC3d comparison, the top 10 significantly enriched GO terms were “procollagen-proline 4-dioxygenase activity”, “peptidyl-proline 4-dioxygenase activity”, “procollagen-proline dioxygenase activity”, “dioxygenase activity”, “protein hydroxylation”, “peptidyl-proline dioxygenase activity”, “oxidoreductase activity, acting on single donors with incorporation of molecular oxygen, incorporation of 2 atoms of oxygen”, “oxidoreductase activity, acting on single donors with incorporation of molecular oxygen”, “oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen, 2-oxoglutarate as one donor, and incorporation of one atom each of oxygen into both donors”, and “extracellular space” (see Figure 3C).
KEGG pathway analysis
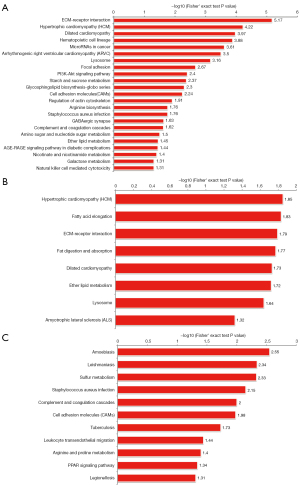
KEGG is a publicly available pathway database that provides biologists with excellent resources to gain a deeper understanding of the biological mechanisms elicited in response to different treatments. According to the KEGG enrichment results for the SC3d vs. SC0d comparison, the 10 top significantly enriched KEGG pathways were the “extracellular matrix (ECM)-receptor interaction”, “hypertrophic cardiomyopathy (HCM)”, “dilated cardiomyopathy”, “hematopoietic cell lineage”, “microRNAs in cancer”, “arrhythmogenic right ventricular cardiomyopathy (ARVC)”, “lysosome”, “focal adhesion”, “phosphatidylinositol-3-kinase (PI3K)-Akt signaling pathway”, and “starch and sucrose metabolism” (see Figure 4A). According to the KEGG enrichment results for the SC7d vs. SC0d comparison, the 10 top significantly enriched KEGG pathways were “HCM”, “fatty acid elongation”, “ECM-receptor interaction”, “fat digestion and absorption”, “dilated cardiomyopathy”, “ether lipid metabolism”, “lysosome”, and “amyotrophic lateral sclerosis (ALS)” (see Figure 4B). According to the KEGG enrichment results for the of SC7d and SC3d comparison, the 10 top significantly enriched KEGG pathways were “amoebiasis”, “leishmaniasis”, “sulfur metabolism”, “staphylococcus aureus infection”, “complement and coagulation cascades”, “cell adhesion molecules (CAMs)”, “tuberculosis”, “leukocyte transendothelial migration”, “arginine and proline metabolism”, and “peroxisome proliferator-activated receptor (PPAR) signaling pathway” (see Figure 4C).
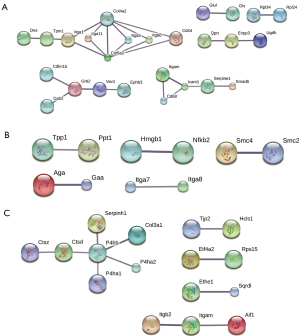
PPI network
The PPI network of the DEPs in the SC3d vs. SC0d comparison contained 26 nodes and 26 connections (see Figure 5A). The PPI network of the DEPs in the SC7d vs. SC0d contained 10 nodes and 5 connections (see Figure 5B). The PPI network of the DEPs in the SC7d vs. SC3d contained 16 nodes and 11 connections (see Figure 5C). The top 5 core genes included Col4a2, Col6a2, Grb2, Icam1, and P4hb. Based on the quantitative data, Col6a2, Grb2, and Icam1 were upregulated, while Col4a2 and P4hb were downregulated.
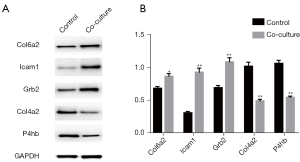
Western blot verification
Western blot was performed to detect the changes in protein levels of the significant DEPs. As Figure 6 shows, compared to the control group, the protein levels of Col6a2, Icam1 and Grb2 were significantly upregulated in the coculture group; however, the protein levels of Col4a2 and P4hb were significantly decreased, which is consistent with the results of the quantitative analysis.
Discussion
SCI is a severe condition characterized by high morbidity and disability. Due to its unknown pathological mechanisms, there has been little progress in the management of SCI to date. Our group has a long-standing interest in SCI treatments based on cell transplantation. In previous research, we demonstrated that SCs can both secrete neurotrophic factors to restrict the apoptosis of neurons and induce BMSCs to differentiate into neuron-like cells, which can promote axonal regeneration and functional recovery (21-24). Several studies have shown that miRNA plays an important role in regulating the neuronal differentiation of stem cells, which are involved in regulating Hippo, Wnt and tumor growth factor-beta (TGF-β) signal pathway (28,29). However, the underlying mechanisms of the interaction between these cells remain unclear. Identifying the molecular mechanism of SCI cell graft therapy was a specific aim of the described project. According to the pathological features of SCI, the efficacy and repair mechanism of co-transplantation SCs and BMSCs at different periods after the injury will be further investigated and defined. The goals of the project were to identify targets and cell signaling pathways and to establish a more reliable and effective treatment strategy for cell transplantation. The outcomes of this proposal will shed light on fundamental problems confounding stem cell therapies and pave the way for further SCI research, increasing the likelihood of early rehabilitation and the efficacy of treatments. iTRAQ is a method with high throughput, stability, and sensitivity to sample properties, and can be used to evaluate the DEPs quantitatively. iTRAQ technology is developed by AB SCIEX in the US, which has been a new tool for quantitative mass spectrometry and widely used in proteome research. In this study, we used the quantitative iTRAQ proteomics to reveal the proteome profiles and investigate the potential mechanisms.
The GO analysis results for the SC3d vs. SC0d comparison show that the “protein complex involved in cell adhesion”, “integrin complex”, “integral component of plasma membrane”, and “integrin-mediated signaling pathway” are significantly enriched pathways. Among the corresponding changes, ICAM1 was upregulated. ICAM1, which is a single-chain cell surface glycoprotein, is a molecule that has significant roles in the inflammatory response and in the recruitment of leukocytes to sites of inflammation (30,31). ICAM1 promotes adhesion at inflammatory sites and regulates the immune response, which is very beneficial in acute SCI. ICAM1 is also believed to be a key factor in inducing angiogenesis, which may ameliorate the ischemia and hypoxia that occur after SCI (32,33).
Integrin and integrin signaling have great significance in axon growth and regeneration in the peripheral nervous system (PNS) and the central nervous system (CNS) (34-36). As a type of transmembrane heterodimeric receptor, integrin may improve bidirectional signaling between the extracellular environment and cells and may have significant roles in cell growth, division, survival, and differentiation (37). Integrin is also important for regulating the coordinated process of leukocyte extravasation into inflammatory sites (38). These three functions are crucial for the repair of SCI. Based on the GO analysis results for the SC7d vs. SC3d comparison, most of the significantly enriched terms are related to dioxygenase activity. It has been reported that MSCs regulate the proliferation, activation, and cytotoxicity related to the immune response via dioxygenase (39). Dioxygenase may also reprogram proinflammatory M1-polarized macrophages toward the anti-inflammatory M2-polarized macrophage phenotype, which is essential for maintaining a balanced microenvironment after damage to the CNS (8,39).
The results of the KEGG pathway analysis revealed some terms related to heart disease and the repair of myocardial cell injury, which are applications of MSCs in cardiac research. Given the similar characteristics of myocardial cells and neurons, this result may provide a meaningful research direction for our focus on SCI. Additionally, “lysosome”, the “PI3K-Akt signaling pathway”, “CAMs”, “leukocyte transendothelial migration”, and “PPAR signaling pathway” may be crucial in the repair of SCI. Lysosome functionality is an important factor in regulating extracellular vesicle (EV) secretion and contents (40). Exosomes are a subtype of EVs, which are vesicles that are 50–100 nm in diameter and mediate intercellular material transfer. Exosomes carry messenger RNAs, micro RNAs and proteins, which can be detected by various techniques (41,42). From 2012 to 2016, Lopez-Verrilli et al. gradually revealed that SC-derived exosomes mediate neuron-glia communication and enhance axonal regeneration in the PNS (43-46). SC-derived exosomes may be involved in a potential mechanism mediating BMSC-stimulated SCI repair. Further, exosomes derived from BMSCs have been shown to promote angiogenesis and axonal regeneration, suppress glial scar formation and inflammation, and improve functional recovery after SCI (47). Exosomes may also mediate material and signaling exchanges between SCs and BMSCs, but this hypothesis requires further study. The PI3K-Akt signaling pathway was reported to regulate human endometrial stem cell differentiation into motor neurons and have a beneficial effect on ischemia/reperfusion injury after SCI (48,49). SCs may promote BMSC differentiation into neuron-like cells and co-grafting these two types of cells can improve functional recovery after SCI via the PI3K-Akt signaling pathway. As mentioned above, “CAMs” and “leukocyte transendothelial migration” play crucial roles in regulating adhesion at inflammatory sites and in the immune response (30,31). It has been reported that PPAR activation can induce anti-inflammatory and antioxidant effects, provide vascular protection, and inhibit apoptosis in the nervous system; thus, PPAR may be a novel pharmacological target in neuroprotection (50-52).
Conclusions
In summary, the mechanisms that occur during the co-transplantation of BMSCs and SCs are complicated and involve a variety of potential signaling pathways that may be related to regulating the inflammatory response, maintaining a balanced microenvironment, promoting angiogenesis and axonal regeneration, improving neuron-like differentiation, secreting neurotrophic factors, and suppressing glial scar formation. Further verification is required to confirm our hypothesis. These results still need to be verified by further experimental work. We believe that our study may provide potential study targets and novel therapeutic directions.
Acknowledgments
Funding: This work was supported by grants from the National Key R&D Program of China (No. 2019YFA0112100) and the Tianjin Key Medical Discipline (Specialty) Construct Project.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3073/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3073/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3073/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. TMUAMEC2017025) granted by the Tianjin Medical University Ethical Committee, in compliance with the Guide for the Care and Use of Laboratory Animals (NIH Publications, revised 2011).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Eli I, Lerner DP, Ghogawala Z. Acute Traumatic Spinal Cord Injury. Neurol Clin 2021;39:471-88. [Crossref] [PubMed]
- Flack JA, Sharma KD, Xie JY. Delving into the recent advancements of spinal cord injury treatment: a review of recent progress. Neural Regen Res 2022;17:283-91. [Crossref] [PubMed]
- Sharma HS, Winkler T. Assessment of spinal cord pathology following trauma using early changes in the spinal cord evoked potentials: a pharmacological and morphological study in the rat. Muscle Nerve Suppl 2002;11:S83-91. [Crossref] [PubMed]
- Choo AM, Liu J, Dvorak M, et al. Secondary pathology following contusion, dislocation, and distraction spinal cord injuries. Exp Neurol 2008;212:490-506. [Crossref] [PubMed]
- Mitchell CS, Lee RH. Pathology dynamics predict spinal cord injury therapeutic success. J Neurotrauma 2008;25:1483-97. [Crossref] [PubMed]
- Ward RE, Huang W, Kostusiak M, et al. A characterization of white matter pathology following spinal cord compression injury in the rat. Neuroscience 2014;260:227-39. [Crossref] [PubMed]
- David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 2011;12:388-99. [Crossref] [PubMed]
- Shechter R, Miller O, Yovel G, et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 2013;38:555-69. [Crossref] [PubMed]
- Hurlbert RJ, Moulton R. Why do you prescribe methylprednisolone for acute spinal cord injury? A Canadian perspective and a position statement. Can J Neurol Sci 2002;29:236-9. [Crossref] [PubMed]
- Schroeder GD, Kwon BK, Eck JC, et al. Survey of Cervical Spine Research Society members on the use of high-dose steroids for acute spinal cord injuries. Spine (Phila Pa 1976) 2014;39:971-7. [Crossref] [PubMed]
- Hurlbert RJ, Hamilton MG. Methylprednisolone for acute spinal cord injury: 5-year practice reversal. Can J Neurol Sci 2008;35:41-5. [Crossref] [PubMed]
- Miekisiak G, Kloc W, Janusz W, et al. Current use of methylprednisolone for acute spinal cord injury in Poland: survey study. Eur J Orthop Surg Traumatol 2014;24:S269-73. [Crossref] [PubMed]
- Druschel C, Schaser KD, Schwab JM. Current practice of methylprednisolone administration for acute spinal cord injury in Germany: a national survey. Spine (Phila Pa 1976) 2013;38:E669-77. [Crossref] [PubMed]
- Khodabandeh Z, Mehrabani D, Dehghani F, et al. Spinal cord injury repair using mesenchymal stem cells derived from bone marrow in mice: A stereological study. Acta Histochem 2021;123:151720. [Crossref] [PubMed]
- Sykova E, Cizkova D, Kubinova S. Mesenchymal Stem Cells in Treatment of Spinal Cord Injury and Amyotrophic Lateral Sclerosis. Front Cell Dev Biol 2021;9:695900. Erratum in: Front Cell Dev Biol 2021;9:770243. [Crossref] [PubMed]
- Lin L, Lin H, Bai S, et al. Bone marrow mesenchymal stem cells (BMSCs) improved functional recovery of spinal cord injury partly by promoting axonal regeneration. Neurochem Int 2018;115:80-4. [Crossref] [PubMed]
- Anderson JD, Johansson HJ, Graham CS, et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells 2016;34:601-13. [Crossref] [PubMed]
- Zeng X, Zeng YS, Ma YH, et al. Bone marrow mesenchymal stem cells in a three-dimensional gelatin sponge scaffold attenuate inflammation, promote angiogenesis, and reduce cavity formation in experimental spinal cord injury. Cell Transplant 2011;20:1881-99. [Crossref] [PubMed]
- Kim GU, Sung SE, Kang KK, et al. Therapeutic Potential of Mesenchymal Stem Cells (MSCs) and MSC-Derived Extracellular Vesicles for the Treatment of Spinal Cord Injury. Int J Mol Sci 2021;22:13672. [Crossref] [PubMed]
- Stratton JA, Shah PT. Macrophage polarization in nerve injury: do Schwann cells play a role? Neural Regen Res 2016;11:53-7. [Crossref] [PubMed]
- Feng SQ, Kong XH, Guo SF, et al. Treatment of spinal cord injury with co-grafts of genetically modified Schwann cells and fetal spinal cord cell suspension in the rat. Neurotox Res 2005;7:169-77. [Crossref] [PubMed]
- Ban DX, Kong XH, Feng SQ, et al. Intraspinal cord graft of autologous activated Schwann cells efficiently promotes axonal regeneration and functional recovery after rat's spinal cord injury. Brain Res 2009;1256:149-61. [Crossref] [PubMed]
- Ban DX, Ning GZ, Feng SQ, et al. Combination of activated Schwann cells with bone mesenchymal stem cells: the best cell strategy for repair after spinal cord injury in rats. Regen Med 2011;6:707-20. [Crossref] [PubMed]
- Zhou XH, Ning GZ, Feng SQ, et al. Transplantation of autologous activated Schwann cells in the treatment of spinal cord injury: six cases, more than five years of follow-up. Cell Transplant 2012;21:S39-47. [Crossref] [PubMed]
- Zhou XH, Lin W, Ren YM, et al. Comparison of DNA Methylation in Schwann Cells before and after Peripheral Nerve Injury in Rats. Biomed Res Int 2017;2017:5393268. [Crossref] [PubMed]
- Spanos C, Moore JB. Sample Preparation Approaches for iTRAQ Labeling and Quantitative Proteomic Analyses in Systems Biology. Methods Mol Biol 2016;1394:15-24. [Crossref] [PubMed]
- Pan D, Li Y, Yang F, et al. Increasing toll-like receptor 2 on astrocytes induced by Schwann cell-derived exosomes promotes recovery by inhibiting CSPGs deposition after spinal cord injury. J Neuroinflammation 2021;18:172. [Crossref] [PubMed]
- Wei ZJ, Fan BY, Liu Y, et al. MicroRNA changes of bone marrow-derived mesenchymal stem cells differentiated into neuronal-like cells by Schwann cell-conditioned medium. Neural Regen Res 2019;14:1462-9. [Crossref] [PubMed]
- Channakkar AS, Singh T, Pattnaik B, et al. MiRNA-137-mediated modulation of mitochondrial dynamics regulates human neural stem cell fate. Stem Cells 2020;38:683-97. [Crossref] [PubMed]
- Liu J, Liu Z, Liu G, et al. Spinal cord injury and its underlying mechanism in rats with temporal lobe epilepsy. Exp Ther Med 2020;19:2103-12. [Crossref] [PubMed]
- Singh M, Thakur M, Mishra M, et al. Gene regulation of intracellular adhesion molecule-1 (ICAM-1): A molecule with multiple functions. Immunol Lett 2021;240:123-36. [Crossref] [PubMed]
- Wang L, Yao Y, He R, et al. Methane ameliorates spinal cord ischemia-reperfusion injury in rats: Antioxidant, anti-inflammatory and anti-apoptotic activity mediated by Nrf2 activation. Free Radic Biol Med 2017;103:69-86. [Crossref] [PubMed]
- Günday M, Saritaş ZK, Demirel HH, et al. Does Anzer Propolis Have a Protective Effect on Rabbit Spinal Cord Ischemia/Reperfusion Injury? Braz J Cardiovasc Surg 2022;37:65-73. [Crossref] [PubMed]
- Ikeshima-Kataoka H, Sugimoto C, Tsubokawa T. Integrin Signaling in the Central Nervous System in Animals and Human Brain Diseases. Int J Mol Sci 2022;23:1435. [Crossref] [PubMed]
- Eva R, Fawcett J. Integrin signalling and traffic during axon growth and regeneration. Curr Opin Neurobiol 2014;27:179-85. [Crossref] [PubMed]
- Sekine Y, Kannan R, Wang X, et al. Rabphilin3A reduces integrin-dependent growth cone signaling to restrict axon regeneration after trauma. Exp Neurol 2022;353:114070. [Crossref] [PubMed]
- Xiong J, Yan L, Zou C, et al. Integrins regulate stemness in solid tumor: an emerging therapeutic target. J Hematol Oncol 2021;14:177. [Crossref] [PubMed]
- Sun H, Hu L, Fan Z. β2 integrin activation and signal transduction in leukocyte recruitment. Am J Physiol Cell Physiol 2021;321:C308-16. [Crossref] [PubMed]
- Zheng G, Ge M, Qiu G, et al. Mesenchymal Stromal Cells Affect Disease Outcomes via Macrophage Polarization. Stem Cells Int 2015;2015:989473. [Crossref] [PubMed]
- Eitan E, Suire C, Zhang S, et al. Impact of lysosome status on extracellular vesicle content and release. Ageing Res Rev 2016;32:65-74. [Crossref] [PubMed]
- Jan AT, Rahman S, Khan S, et al. Biology, Pathophysiological Role, and Clinical Implications of Exosomes: A Critical Appraisal. Cells 2019;8:99. [Crossref] [PubMed]
- Dilsiz N. Hallmarks of exosomes. Future Sci OA 2022;8:FSO764. [Crossref] [PubMed]
- Lopez-Verrilli MA, Court FA. Transfer of vesicles from schwann cells to axons: a novel mechanism of communication in the peripheral nervous system. Front Physiol 2012;3:205. [Crossref] [PubMed]
- Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 2013;61:1795-806. [Crossref] [PubMed]
- López-Leal R, Alvarez J, Court FA. Origin of axonal proteins: Is the axon-schwann cell unit a functional syncytium? Cytoskeleton (Hoboken) 2016;73:629-39. [Crossref] [PubMed]
- Lopez-Leal R, Court FA. Schwann Cell Exosomes Mediate Neuron-Glia Communication and Enhance Axonal Regeneration. Cell Mol Neurobiol 2016;36:429-36. [Crossref] [PubMed]
- Liu W, Wang Y, Gong F, et al. Exosomes Derived from Bone Mesenchymal Stem Cells Repair Traumatic Spinal Cord Injury by Suppressing the Activation of A1 Neurotoxic Reactive Astrocytes. J Neurotrauma 2019;36:469-84. [Crossref] [PubMed]
- Ebrahimi-Barough S, Hoveizi E, Yazdankhah M, et al. Inhibitor of PI3K/Akt Signaling Pathway Small Molecule Promotes Motor Neuron Differentiation of Human Endometrial Stem Cells Cultured on Electrospun Biocomposite Polycaprolactone/Collagen Scaffolds. Mol Neurobiol 2017;54:2547-54. [Crossref] [PubMed]
- Zhang F, Ru N, Shang ZH, et al. Daidzein ameliorates spinal cord ischemia/reperfusion injury-induced neurological function deficits in Sprague-Dawley rats through PI3K/Akt signaling pathway. Exp Ther Med 2017;14:4878-86. [Crossref] [PubMed]
- Wnuk A, Kajta M. Steroid and Xenobiotic Receptor Signalling in Apoptosis and Autophagy of the Nervous System. Int J Mol Sci 2017;18:2394. [Crossref] [PubMed]
- Mannan A, Garg N, Singh TG, et al. Peroxisome Proliferator-Activated Receptor-Gamma (PPAR-ɣ): Molecular Effects and Its Importance as a Novel Therapeutic Target for Cerebral Ischemic Injury. Neurochem Res 2021;46:2800-31. [Crossref] [PubMed]
- Toobian D, Ghosh P, Katkar GD. Parsing the Role of PPARs in Macrophage Processes. Front Immunol 2021;12:783780. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


