
Successful secondary radical operation on unretractable metastatic platinum-sensitive recurrent ovarian cancer by immunotherapy: a case report
Introduction
Recurrent ovarian cancer (ROC) is taken for a uncurable disease and following recurrence, chemotherapy regimens, including platinum agents, are mainly used to prolonging life (1,2). Current treatment options for patients with ROC depend on their response to first-line platinum-based chemotherapy, with patients classified as platinum-sensitive, partially platinum-sensitive, and platinum-resistant, as measured by the platinum-free interval (PFI) (3). Carboplatin is one of the most effective chemotherapy drugs for ovarian cancer, but only 30–60% of platinum-sensitive ROC response to further platinum (4). And these platinum-sensitive patients with multidrug resistance (MDR) are usually managed with non-platinum chemotherapy such as doxorubicin. Moreover, although the survival benefit of ROC reoperation is controversial, several studies have shown that complete secondary reduction surgery is a key predictor of survival for ROC (5-7). The prospective data obtained from the AGO DESKTOP-III trial (NCT01166737), which is the first surgical study to show a meaningful survival benefit in ROC, found that patients with ovarian cancer who undergo a 1st relapse after a 6-month PFI could benefit from secondary cytoreductive surgery (8). However, there is no standard treatment for unretractable platinum-sensitive ROC when conventional therapy is ineffective, especially for patients with quick disease progression and serious adverse events from 1st line therapy (9). The development of immune drugs has opened up many options for conversion therapy in advanced cancer. Immune checkpoint blockades, such as programmed cell death protein 1 (PD-1) inhibitor or programmed death-ligand 1 (PD-L1) inhibitor, work by blocking inhibitory receptors on T cells or their ligands, preventing T cells from exhaustion and intensifying their ability to detect and destroy tumors (10). But so far, immunotherapy has not been as successful in treating ovarian cancer as it has been in other cancers. The knowledge of ovarian cancer biology is key to developing known immunotherapy strategies (11). In this article, we present the case of a multiple metastases unresectable platinum-sensitive ROC patient with resistant platinum-based chemotherapy at 1st line and invalid to non-platinum-based chemotherapy at 2nd line, who had benefited from PD-1inhibitor combination therapies rely on immune-infiltrating tumor microenvironment signatures rather than established immunotherapy biomarkers, and who successfully underwent a secondary radical operation. We present the following case in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2128/rc).
Case presentation
A 57-year-old female patient, 64 kg, married, no special diet, without familial cancer history, has a history of arrhythmia and chronic bronchitis. She underwent surgery (total uterus, double appendages, omentum, appendix resection, and pelvic, para-aortic, and presacral lymph node dissection) and 6 cycles of platinum-containing adjuvant chemotherapy for primary ovarian cancer in October 2015. She presented complaining of spontaneous pain from the right subcostal margin for 1 month and was diagnosed with ROC in April 2019. Multiple liver metastases (maximum 4.5×4.0 cm2) and a large vaginal stump bump (6.5×4.8 cm2) were observed by computed tomography (CT) after a postoperative 36.5-month period of no adjuvant treatment (see Figure 1). Additionally, a colonoscopy showed an ulcer-like mass about 25 cm from the anus, blocking most of the intestinal cavity, which was thought to be high-grade serous ovarian cancer (HGSOC) based on the pathology biopsy results.
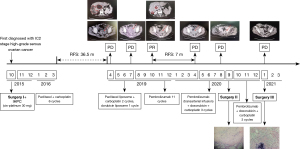
Standard systemic chemotherapy regimens had failed successively since May 2019. Standard chemotherapy with paclitaxel (330 mg) combined with carboplatin (650 mg) had been performed every 3 weeks (Q3W) as an initial treatment. However, the patient exhibited further growth in the vaginal stump bump and multiple cancer foci on the surface of the sigmoid colon (a part of the transverse colon), and the mesentery of the small intestine coupled with grade IV myelosuppression and intestinal obstruction, which led to the prescription of 20 mg of liposomal doxorubicin single-agent chemotherapy (D1, D8). The further deterioration of gastrointestinal function led to extreme debilitation and chemotherapy drug intolerance, and the hepatic metastasis covered almost 2/3 of the total liver volume.
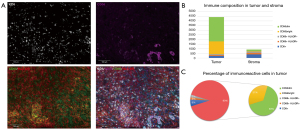
To find a more appropriate medication regimen, the tumor-related information was analyzed. Next-generation sequencing (NGS) of a plasma sample using a 733-gene panel identified a low tumor mutational burden and microsatellite stability (MSS), and no mutation in the breast cancer 1 (BRAC1) or breast cancer 2 (BRCA2) gene (see Figure 2). However, obvious immune infiltration into the tumor via an examination of the tumor immune microenvironment using the pelvic tumor samples of the patient was observed, the expression of programmed death-ligand 1 (PD-L1) was negative in the tumor cells (see Figure 3A,3B). Among the lymphocytes that infiltrated the tumor, natural killer (NK) cells accounted for a 90% proportion of the lymphocytes (see Figure 3C).
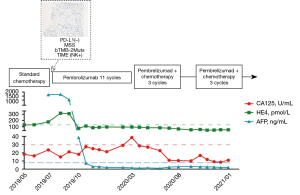
Intravenous (i.v.) injection of pembrolizumab (i.v. 2 mg/kg, Q3W) was started on August 23, 2019, as no other more viable and efficacious treatment options were available. After 3 periods of treatment, the patient achieved first partial response (PR) and significant tumor reduction in both the liver and the pelvis. Consistent with the CT results, the tumor markers of human epididymis protein 4 (HE4) and alpha-fetoprotein (AFP) showed a dramatic decrease to normal levels.
In April 2020, the ovarian cancer marker, cancer antigen 125 (CA125), rose to 38.6 U/mL, and the retroperitoneal lymph node was observed to be enlarged. The CT scan also showed the continuous growth of the pelvic tumor (8.3×7.3 cm2), which indicated tumor progression again. Given that the patient might have developed immune resistance, we subsequently switched the patient from pembrolizumab monotherapy to pembrolizumab plus chemotherapy [interval combined with doxorubicin liposomes (i.v. 40 mg, D1) and carboplatin (i.v. 550 mg, D2)] and changed the drug regimen of pembrolizumab (200 mg, percutaneous right femoral artery puncture of the inferior mesenteric artery + left iliac artery perfusion). On August 10, CA125 dropped to the normal level (i.e., <35 U/mL), and CT scans revealed shrinkage of the pelvic tumors by 50%. Because of the conversion immunotherapy, a reduction in the extent of the intra-abdominal disease, and an improvement in the general condition of the patient was observed. Pelvic floor mass resection, rectal resection, and partial sigmoidectomy were achieved by R0 resection. The postoperative pathology results showed the following proteins/markers: AE1/AE3(3+), CK18(2+), CK7(3+), PAX8(–), P40(1+), S-100(1+), mut-P53, P16(3+), WT-1(–), ER(–), PR(–), Ki-67(50%), desmin(–) protein, and integrase interactor 1 (INI1)(3+). The pathology results suggested the presence of malignant tumors in the colon wall (rectum and partial sigmoid colon), consistent with the recurrence of HGSOC with local chondrosarcomatoid differentiation. The patient felt better and recovered well after surgery, and postoperative pembrolizumab combined with chemotherapy adjuvant therapy was continued. In January 2021, the CT scans revealed no tumor lesion in the pelvis, and the surgery of the R0 resection of liver metastases was successfully performed. From then until press time, the patient made a good recovery and disease free for at least 17 months. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Platinum-based therapy, non-platinum-based therapy and surgery are considered standard options for platinum-sensitive relapse. Platinum-sensitive ROC refers to a PFI of at least 6 or 12 months, and these patients tend to show better treatment responses and prognoses than their platinum-resistant counterparts (12,13). Although these definitions, platinum-refractory, resistant, partially sensitive, and sensitive, have been used to identify different ROC populations, the resistance to platinum-based treatment is not a categorical variable (14). This patient with TP53 mutation (p.V272del) which could increase resistance to various DNA-damaging agents (including platinum) via reducing the sensitivity of cells to activate apoptotic responses (15). Secondary cytoreduction in platinum-sensitive ROC remains controversial because of the mixed results in terms of the overall survival (OS) benefit. A systematic review displayed an increased OS in platinum-sensitive patients who under cytoreduction had no residual macroscopic (R0) disease compared to those who had any visual disease (hazards ratio =3.59, 95% confidence interval: 2.45–5.34) (16). Recently, the DESKTOP-III (NCT01166737) and SOC-1 trials (NCT01611766), 2 randomized phase-III trials examining the use of secondary cytoreduction in ROC patients, achieved a high rate of complete gross resection (75% in DESKTOP-III and 77% in SOC-1) and a survival benefit (17). In our case, secondary surgical debulking for unretractable ROC was successfully performed via immunotherapy strategies under the chemotherapy intolerance.
There are a number of clinical trials trying to find a niche for different immunotherapy approaches. Although the relevance of cancer microenvironments becomes clearer, there is no guideline to address the optimal immunotherapy strategy to use according to different tumor microenvironments. A meta-analysis showed that CD8 TILs and the immunoreactive HGSOC are associated with the BRCA1 mutation and not with BRCA2 mutation (18). Interestingly, the patient was found an unknown clinical significance of BRAC1 mutation (p.K711E) and significantly infiltrated with the CD8 T cells and NK cells. Chemotherapy also could induce immunomodulatory effects. Some studies indicated that certain chemotherapeutic agents might stimulate immunogenic cell death, a type of apoptosis that induces immune response (9,19).
According to observations on the anti-PD-1 therapeutic benefits of hematologic malignancies, the use of anti-PD-1 agents following chemotherapy might lead to a more robust tumor-specific response during the period of immune reconstitution when the bone marrow recovers from the myelosuppression by cytotoxic agents (20). Multiple fluorescence immunohistochemistry (MFI) was performed to further investigate the possible reasons for the benefit of immunotherapy in this patient. Despite the patient being MSS according to the genetic testing, MFI revealed a high filtration level of NK cells and cluster of differentiation CD8T cells in the tumor tissue. Several studies on the significance of the tumor immune microenvironment in the prognosis of ovarian cancer have shown that the presence of intra-tumoral T cells is associated with better clinical outcomes (21,22). NK cells are important innate immune cells, which play a key role in the early anti-tumor immune response by directly killing tumor cells (23).
The neural cell adhesion molecule (NCAM, or CD56), which is an important marker found on the surface of NK cells, can be used to identify NK cells. According to the expression intensity of CD56, NK cells can be divided into CD56dim (weak staining) and CD56bright (strong staining) (24). CD56dim has stronger killing activity than CD56bright. Several studies have shown that NK cell infiltration has some implications for the treatment and prognosis of tumors. A clinical study of 145 patients with head and neck squamous cell carcinoma who underwent surgery and postoperative chemoradiotherapy showed that patients with a higher number of infiltrating NK cells had longer OS (P=0.0001) and a lower risk of distant recurrence (P=0.0001) (25). A significant number of infiltrating NK cells was also associated with a better prognosis of immunotherapy. Melanoma patients treated with nivolumab, with a higher level of NK cell infiltration and elevated CD8+ T cells in tumor tissues, displayed better clinical responses at 4 weeks after treatment (26).
Cytotoxic T cells are important immune cells in the human immune system and can eliminate tumor cells by directly killing them. A study of 46 melanoma patients treated with pembrolizumab showed that the density of CD8+ T cells at the aggressive edge of the tumor before treatment was higher in the tumor tissue of responders than in the tumor tissue of non-responders. During the course of treatment, the density of CD8+ T cells in the tumor parenchyma and the aggressive margins of the tumor were further increased in responders (27). A study suggested that the density of CD8+ T cells in the tumor tissues of melanoma patients who responded to a PD-1 monoclonal antibody was higher than that in the tumor tissues of melanoma patients who did not respond to a PD-1 monoclonal antibody, and the density of CD8+ T cells increased more significantly in patients who responded to the PD-1 monoclonal antibody during treatment (28).
Notably, the transarterial infusion of a pembrolizumab interval combined with the intravenous use of doxorubicin and carboplatin was effective in addressing immune resistance when disease progression occurred again after nearly 9 months of pembrolizumab monotherapy. According to previous studies of arterial infusion chemotherapy for ovarian cancer (29) and transarterial infusion of pembrolizumab for liver metastases of melanoma (30), the intra-arterial administration of pembrolizumab offers a much higher local drug concentration than the intravenous administration. Additionally, the immunostimulatory effects of chemotherapeutic drugs have already been proven to activate effector cells and/or inhibit immunosuppressive cells or enhance immunogenicity and improve the infiltration of lymphocytes (31). For example, gemcitabine or a small dose of 5-FU, could increase the efficacy of cell immunotherapy by regulating myeloid-derived suppressor cells, and oxaliplatin might induce novel T-cell infiltration of the tumor, etc. These findings indicate the feasibility of using immune checkpoint inhibitors combined with chemotherapy to improve drug resistance (32). Regulation of immune cells in tumor microenvironment by chemotherapy drugs might partly explain why the immunotherapy combination strategies worked well.
In conclusion, this case report revealed immune infiltration signatures could use as an indicator of immunotherapy when all approved biomarkers negatived for ROC patients, especially those with MDR platinum-sensitive ROC. And secondly the patient’s effective tumor response provided an opportunity for the 2nd radical surgery, which result in longer-term survival benefits. For future clinical trials and research, it might be advisable to evaluate the reliability of immune cell infiltration as a predictor of immunotherapy to inform treatment decisions for this sub-population.
Acknowledgments
We would like to thank Miao Liu, Dandan Fan, Yaoxu Chen, Chan Gao, and Mengli Huang from The Medical Department, 3D Medicines Inc. for their valuable help in the editing of this manuscript and for their support in investigating the multiple fluorescence immunohistochemistry of the tumor samples.
Funding: The research was supported by the Sanming Project of Medicine in Shenzhen (No. SZSM201812075).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2128/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2128/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cancer Care Ontario and American Society of Clinical Oncology Adjuvant Chemotherapy and Adjuvant Radiation Therapy for Stages I-IIIA Resectable Non-Small-Cell Lung Cancer Guideline. J Oncol Pract 2007;3:332-5. [Crossref] [PubMed]
- Casado A, Callata HR, Manzano A, et al. Trabectedin for reversing platinum resistance and resensitization to platinum in patients with recurrent ovarian cancer. Future Oncol 2019;15:271-80. [Crossref] [PubMed]
- Wilson MK, Pujade-Lauraine E, Aoki D, et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recurrent disease. Ann Oncol 2017;28:727-32. [Crossref] [PubMed]
- Sehouli J, Alfaro V, González-Martín A. Trabectedin plus pegylated liposomal doxorubicin in the treatment of patients with partially platinum-sensitive ovarian cancer: current evidence and future perspectives. Ann Oncol 2012;23:556-62. [Crossref] [PubMed]
- Sánchez-Iglesias JL, Gómez-Hidalgo NR, Pérez-Benavente A, et al. Importance of Enhanced Recovery After Surgery (ERAS) Protocol Compliance for Length of Stay in Ovarian Cancer Surgery. Ann Surg Oncol 2021;28:8979-86. [Crossref] [PubMed]
- Nelson G, Bakkum-Gamez J, Kalogera E, et al. Guidelines for perioperative care in gyneco logic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations—2019 update. Int J Gynecol Cancer 2019;29:651-68. [Crossref] [PubMed]
- Sanchez-Iglesias JL, Carbonell-Socias M, Perez-Benavente MA, et al. PROFAST: A randomised trial implementing enhanced recovery after surgery for high-complexity advanced ovarian cancer surgery. Eur J Cancer 2020;136:149-58. [Crossref] [PubMed]
- Bois AD, Sehouli J, Vergote I, et al. Randomized phase III study to evaluate the impact of secondary cytoreductive surgery in recurrent ovarian cancer: Final analysis of AGO DESKTOP III/ENGOT-ov20. J Clin Oncol 2020;38:6000. [Crossref]
- Moya-Alarcón C, Piera G, Callejo Á, et al. Real-world treatment patterns and outcomes in platinum-sensitive recurrent high-grade serous ovarian cancer patients. J Comp Eff Res 2022;11:13-27. [Crossref] [PubMed]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1-10. [Crossref] [PubMed]
- García-Martínez E, Pérez-Fidalgo JA. Immunotherapies in ovarian cancer. EJC Suppl 2020;15:87-95. [Crossref] [PubMed]
- Friedlander M, Trimble E, Tinker A, et al. Clinical trials in recurrent ovarian cancer. Int J Gynecol Cancer 2011;21:771-5. [Crossref] [PubMed]
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: A review. Gynecol Oncol 2019;152:416-25. [Crossref] [PubMed]
- Pignata S, C, Cecere S, Du Bois A, et al. Treatment of recurrent ovarian cancer. Ann Oncol 2017;28:viii51-6. [Crossref] [PubMed]
- Lavarino C, Pilotti S, Oggionni M, et al. p53 gene status and response to platinum/paclitaxel-based chemotherapy in advanced ovarian carcinoma. J Clin Oncol 2000;18:3936-45. [Crossref] [PubMed]
- Al Rawahi T, Lopes AD, Bristow RE, et al. Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst Rev 2013;CD008765. [Crossref] [PubMed]
- Richardson DL. Should we or should we not? Secondary debulking in ovarian cancer. Lancet Oncol 2021;22:412-3. [Crossref] [PubMed]
- Clarke B, Tinker AV, Lee CH, et al. Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol 2009;22:393-402. [Crossref] [PubMed]
- Wang Q, Ju X, Wang J, et al. Immunogenic cell death in anticancer chemotherapy and its impact on clinical studies. Cancer Lett 2018;438:17-23. [Crossref] [PubMed]
- Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol 2013;31:4199-206. [Crossref] [PubMed]
- Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003;348:203-13. [Crossref] [PubMed]
- Webb JR, Milne K, Watson P, et al. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res 2014;20:434-44. [Crossref] [PubMed]
- Watkins-Schulz R, Tiet P, Gallovic MD, et al. A microparticle platform for STING-targeted immunotherapy enhances natural killer cell- and CD8+ T cell-mediated anti-tumor immunity. Biomaterials 2019;205:94-105. [Crossref] [PubMed]
- Poli A, Michel T, Thérésine M, et al. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 2009;126:458-65. [Crossref] [PubMed]
- Stangl S, Tontcheva N, Sievert W, et al. Heat shock protein 70 and tumor-infiltrating NK cells as prognostic indicators for patients with squamous cell carcinoma of the head and neck after radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int J Cancer 2018;142:1911-25. [Crossref] [PubMed]
- Riaz N, Havel JJ, Makarov V, et al. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell 2017;171:934-949.e16. [Crossref] [PubMed]
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. [Crossref] [PubMed]
- Chen PL, Roh W, Reuben A, et al. Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov 2016;6:827-37. [Crossref] [PubMed]
- Aigner KR, Selak E, Gailhofer S, et al. Hypoxic Isolated Abdominal Perfusion (HAP) chemotherapy for non-operable advanced staged ovarian cancer with peritoneal carcinosis: an experience in 45 platinum-refractory ovarian cancer patients. Indian J Surg Oncol 2019;10:506-14. [Crossref] [PubMed]
- Shen L, Qi H, Chen S, et al. Cryoablation combined with transarterial infusion of pembrolizumab (CATAP) for liver metastases of melanoma: an ambispective, proof-of-concept cohort study. Cancer Immunol Immunother 2020;69:1713-24. [Crossref] [PubMed]
- Heinhuis KM, Ros W, Kok M, et al. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol 2019;30:219-35. [Crossref] [PubMed]
- Patel SA, Minn AJ. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity 2018;48:417-33. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


