
Midkine promotes kidney injury in diabetic kidney disease by increasing neutrophil extracellular traps formation
Introduction
It was estimated that the global prevalence of diabetes in individuals aged 20–79 years in 2021 was 10.5% (536.6 million people), and will rise to 12.2% (783.2 million) in 2045 (1). Diabetic kidney disease (DKD) is the most common microvascular complication of diabetes (2,3). It is the primary cause of the end-stage renal disease (ESRD) in European and American countries, and the 2nd cause of ESRD in China (4). In 2016, a multi-center study in China confirmed that DKD had surpassed chronic glomerulonephritis to become the 1st cause of hospitalization for chronic kidney disease patients in China (5). DKD was characterized by proteinuria, thickening of the renal basement membrane, expansion of mesangial area, continuous renal fibrosis, and progressive renal hypofunction (6,7). The diminished renal function caused by DKD would not only shorten the life expectancy but also increase the risk of cardiovascular disease of patients, which will seriously affect the quality of life (8). However, there is no specific effective drug for the treatment of DKD presently. The pathogenesis of DKD was complex and involved many factors, including hemodynamic disorder, glucose and lipid metabolism disorders, advanced glycosylation end products (AGEs), advanced glycosylation end products (ALEs) accumulation, renin-angiotensin-aldosterone system (RAAS) activation, oxidative stress reaction, and so forth (9). Thus, an in-depth exploration of the pathogenesis of DKD is of important clinical significance if effective treatments are to be developed.
In recent years, the role of chronic inflammation in the progression of DKD has been the subject of increasing research (10). Neutrophils are the largest number of white blood cells in the peripheral blood and play an extremely critical role in the immune response. In 2004, Brinkmann et al. (11) discovered for the first time that activated neutrophils participate in capturing and killing pathogenic bacteria by releasing neutrophil extracellular traps (NETs). NETosis refers to the way neutrophils form NETs. NETs are a new pathway of neutrophil death that are different from apoptosis and necrosis. NETs are composed of DNA and various proteins including neutrophil elastase, citrullinated histones (CitH3), neutrophil elastase (NE), myeloperoxidase (MPO), and other proteins. NETs wrap and kill invading pathogens (12). However, NETosis is also a “double-edged sword”. If NETs are over-formed or not cleared in time, this gummy network structure, which is rich in hydrolase and DNA, can attach to the vascular endothelium, causing endothelial cell apoptosis and tissue damage. At the same time, it can also induce autoimmunity by releasing endogenous danger signals (13). Research has shown that NETosis promotes the progression of various kidney diseases, including post-infection glomerulonephritis, renal ischemia-reperfusion injury, antineurophil cytoplasmic antibody (ANCA)-related vasculitis, lupus nephritis, anti-basement membrane nephropathy, and membranous glomerulonephritis through direct pro-inflammatory effects aseptic necrotizing inflammation, thrombosis, and the generation of autoantibodies against NETs (14). Additionally, NETosis has also been reported to facilitate the occurrence and development of diabetes and its complications (15). Neutrophils are critical component of the innate immune system which offer frontline defense against pathogens through a variety of potent effector functions. Uncontrolled activation of neutrophils leads to the production of reactive oxygen species (ROS) and NETs that play important roles in type-2 diabetes and atherosclerosis. One possibility is the activation of Toll-like receptors (TLRs) from the byproducts on persistent sterile inflammation (16). However, the mechanism of NETosis in the pathogenesis of DKD needs to be further explored.
Midkine (MK) is a heparin-binding cytokine, also known as a growth factor, with a molecular weight of 13 kD, which has a number of functions, including the chemotaxis of inflammatory cells, anti-damage, and anti-apoptosis (17). Under physiological conditions, the MK gene is highly expressed in embryonic epithelial-mesenchymal tissues and nerve tissues; however, it is almost never expressed in other parts, including brain, heart, liver, lungs, and fatty tissue, except the kidney, testis, stomach, and small intestinal epithelium in adult tissues (18). Upon binding to its receptors, MK activates multiple signal pathways to regulate cell survival and migration, epithelial-to-mesenchymal transition, and oncogenesis (19). Recent research has shown that in the heart tissue of myocarditis patient and mouse models, MK induces neutrophil migration and NETosis formation through the low-density lipoprotein receptor 1, and promotes the occurrence of myocarditis. Targeting MK protects heart function by reducing the formation of NETosis (20). Further, MK is closely related to a variety of kidney diseases (21). It was reported that MK is a biomarker for early detection of diabetic nephropathy in children with type 1 diabetes mellitus (T1DM) (22). In Mdk−/− mice, nephropathy was strikingly milder than Mdk+/+ mice after Streptozotocin injection (23). Meanwhile, MK promoted tubulointerstitial inflammation in the diabetic mouse model by activating the MCP-1 signaling pathway (24). However, it is unclear whether MK participates in the occurrence and progression of DKD by promoting NETosis. This study sought to investigate whether MK participates in DKD through NETosis using in-vitro kidney tissue samples from DKD patients and cell cultures, and in-vivo type 2 diabetes mouse models, and to provide a new theoretical basis for the prevention and treatment of clinical DKD. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2382/rc).
Methods
Clinical samples collection
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ethics committee of Shanxi Provincial People’s Hospital (Taiyuan, China) granted approval for the experiment procedure (No. 2022-58). The patients are all from Shanxi Provincial People’s Hospital. Renal tissue biopsy samples were obtained from 10 DKD patients and adjacent normal renal tissues of 10 renal cancer patients via nephrectomy were used as controls. The kidney tissues were subject to pathological examination, immunohistochemistry (IHC), and immunofluorescence (IF) staining. All the patients provided written informed consent for this study.
Neutrophil isolation and identification
Animal experiments were carried out following a protocol approved by the Animal Ethics Committee of Shanxi Bethune Hospital (No. SBQKL-2021-028), in compliance with Shanxi Bethune Hospital guidelines for the care and use of animals. A protocol was prepared before the study without registration. The bone marrow of the femur and tibia was collected from healthy male C57BL/6 mice and washed with 1×hanks balanced salt solution (HBSS, containing 1% bovine serum albumin (BSA), without Ca2+ and Mg2+). Next, the filtrate was collected and centrifuged for 10 min × 500 g at 4 ℃ to obtain the bone marrow cells. The neutrophils were isolated from the bone marrow by density gradient centrifugation as previously described (25). Flow cytometry was performed to identify isolated neutrophils (Ly6G+CD11b+). The antibodies used were anti-mouse Ly6G (clone 1A8, 127625, BioLegend), and anti-mouse/human CD11b (clone M1/70, 101204, BioLegend). Following 1 h of incubation at 4 ℃, the samples were run on a BD FACSCanto II flow cytometer. Subsequently, the neutrophils were seeded into 4-well cell culture sections at a density of 2×105/well and incubated for 1 h. The cells were fixed with 95% ethanol for 20 min, stained with wright staining solution (Solarbio, China), and observed using a microscope (Olympus Corporation, Japan).
CCK-8 assays
The neutrophils were cultured in 96-well plates with an inoculation density of 1×104/well for 24 h at 37 ℃ in 5% carbon dioxide. Next, the neutrophils were cultured for a further 24, 48, or 72 h after being treated with different concentrations of MK (CYT-178, ProSpec, Israel). The cell counting kit-8 (CCK-8, BS350B, Biosharp, China) was used to determine the proliferation of cells according to the operating manual. The absorbance was recorded at 450 nm by a microplate reader (ThermoFisher Scientific, USA).
For the in-vitro experiment, the neutrophils were divided into the following 6 groups: (I) the normal-glucose (NG) group (which received 5.5 mM of D-glucose); (II) the NG + MK group (which received 5.5 mM of D-glucose + 100 ng/mL of MK); (III) the mannitol group (MG; a hyperosmolar control; which received 5.5 mM of D-glucose + 25 mM of mannitol); (IV) the MG + MK group (which received 5.5 mM of D-glucose + 25 mM of mannitol + 100 ng/mL of MK); (V) the high-glucose (HG) group (which received 25 mM of D-glucose); and (VI) the HG + MK group (which received 25 mM of D-glucose + 100 ng/mL of MK). The concentration of MK used in cell experiment referenced previously published article (26).
Animals experiment
Healthy male C57BL/6 mice (weighing 15–25 g and aged 6 weeks old) were provided by the Experimental Animal Center of Shanxi Medical University. The temperature and relative humidity of the rearing room was 25±2 ℃ and 40% to 60%, respectively. All the mice were supplied with standard diet water ad libitum.
The 30 mice were randomly divided into the following 3 groups (n=10): (I) the normal control (NC) group; (II) the diabetes mellitus (DM) group; and (III) the DM + MK antisense oligodeoxynucleotide group (DM + MK – ODN). The DKD model with DM was induced by a high-fat diet for 4 consecutive weeks (60 kcal% Fat, D12492, Dowsontec, China), and an intraperitoneal injection of 40 mg/kg/d of STZ (Sigma Aldrich, USA) (dissolved in citrate buffer, pH 4.0) after overnight fasting for 3 consecutive days (27). Mice with fasting blood glucose (FBG) levels ≥11.1 mM were regarded as type 2 diabetic mice 7 days after injection (28). From the 9th week, 20 µL of MK antisense oligodeoxynucleotide (ODN; 1 mM, 5'-AGGGCGAGAAGGAAGAAG-3', corresponding to bases 15 to 32 in MK complementary DNA) and 30 µL of Plus reagent (Invitrogen) were mixed in phosphate-buffered saline (PBS; total: 100 µL). Next, this mixture was administered via a tail vein injection in the DM + MK – ODN group once a week for 8 consecutive weeks (29). The mice in the DM group were injected with equivalent volume of Plus reagent (Invitrogen) and PBS. Additionally, mice in the NC group were supplied with a normal diet and the same volume of citrate buffer and saline. Urine was accurately collected for 24 h in the metabolic cage 1 day before tissue collection. Blood was taken from the orbit of the mice under anesthesia with sodium pentobarbital (40 mg/kg). Serum and urine were stored at –80 ℃ for further assays. Kidneys were collected for the histological analysis and western blot assays.
Renal pathological examination
The kidney tissues of the clinical specimens and DM mice were dissected, fixed, dehydrated, embedded, and cut into 3-µm sections, stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), and Masson, separately. The stained sections were analyzed using a digital trinocular camera microscope (Panthera, Motic China Group Co., Ltd., China) and image analysis software Image-Pro Plus 6.0 (Media Cybernetics, USA).
General biochemical parameters
At 24 h, urine albumin was measured by radioimmunoassay (Furui, Beijing, China). The levels of FBG, serum creatinine (Scr), and urine creatinine (Ucr) were measured with an automatic biochemical analyzer (model 7150; Hitachi, Tokyo, Japan), and the level of the urine albumin/creatinine ratio (UACR) was analyzed (in mg albumin/g of creatinine).
ELISA
The levels of NE, MK, interleukin (IL)-1, and IL-6 from the DM mouse serum samples or neutrophil supernatant were examined using the ELISA kit (ZhuoCai Biological Technology, China) in accordance with the manufacturer’s instructions. The serum samples were added into NE, MK, IL-1, or IL-6 pre-coated wells. Next, the anti-hamster (NE, MK, IL-1, or IL-6) monoclonal antibodies were added, respectively. These antibodies were labeled with biotin and combined with Streptavidin-horseradish peroxidase (HRP) to form an immune complex. After the immune complex was treated as described, the absorbance of the wells was measured with a microplate reader (SpectraMAX Plus384, USA) at a 450-nm wavelength to calculate the sample concentration in accordance with the manufacturer’s instructions.
Western blot analysis
Total protein was collected from the kidneys of the DM mice or neutrophils using a Total Protein Extraction Kit (BC3711, Solarbio) and quantified with a BCA protein quantification kit (ab102536, Abcam, Cambridge, MA, UK) in accordance with the operating manual. Protein samples were electrophoresed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) at 80 V for 30 min and 110 V for 50 min and then transferred to a polyvinylidene fluoride (PVDF) membrane electrically. The membraneswere the pre-blocked with Tris-buffered saline solution/ 0.1% Tween (TBST; Sigma Aldrich, USA; containing 5% skim milk) for 1 h at room temperature and incubated with primary myeloperoxidase (MPO) antibody (1:1,000, ab208670, Abcam, Cambridge, MA, UK), histone H3 (citrulline R2 + R8 + R17) antibody (1:1,000, ab5103, Abcam, Cambridge, MA, UK), α-smooth muscle actin antibody (α-SMA; 1:1,000, 19245, Cell Signaling, Danvers, MA), collagen I antibody (1:1,000, ab34710, Abcam, Cambridge, MA, UK), collagen IV antibody (1:1,000, ab6586, Abcam, Cambridge, MA, UK), fibronectin 1 (FN1; 1:1,000, 2413, Cell Signaling, Danvers, MA), and β-actin antibody (1:1,000, AC026, Abclonal, USA) at 4 ℃ overnight. After washing the membrane with TBST, the membrane was hatched with goat-anti-rabbit immunoglobulin G (IgG) (H + L)-HRP (1:10,000, ab6721, Abcam, Cambridge, MA, UK) for 1 h at 37 ℃ and then washed with TBST. The visualization of the reaction was developed by 3,3-diaminobenzidine (DAB; Sigma, USA) for 10 min.
IHC
The kidney samples of the clinical specimens and DM mice were fixed with 4% paraformaldehyde at room temperature for 5 h before being embedded in paraffin. Next, the paraffin sections (5 µm) were used for the IHC assays. The sections were stained by rabbit polyclonal MK antibody (1:100, bs-1849R, Bioss, China). Biotinylated goat anti-rabbit IgG (SP9001, ZsBio, China) was used as a secondary antibody. The stained sections were analyzed using the digital trinocular camera microscope (Panthera, Motic China Group Co., Ltd., China) and image analysis software Image-Pro Plus 6.0 (Media Cybernetics, USA).
IF of the formation of NETosis
Paraffin sections of kidney tissues from the clinical specimens and DM mice were dewaxed and hydrated before staining. The sections were incubated in QuickBlock™ Blocking Buffer (Beyotime Biotechnology, Jiangsu, China, P0260) for 30 min at room temperature. The sections were then incubated with MPO antibody (Abclonal, China, A1374, 1:100) or H3Cit antibody (Biyuntian, China, AF0009, 1:100) at 4 ℃ overnight and washed 3 times with PBS. Fluorescein isothiocyanate (FITC) goat anti-rabbit IgG or Cy3-conjugated goat anti-mouse IgG was used as a secondary antibody. 4',6-diamidino-2-phenylindole (DAPI; Abcam, ab104139; 1/2,000) was added dropwise into the sections for 5 min. The staining was observed under a fluorescence microscope BX53 (Olympus, Tokyo, Japan) at ×100 magnifications.
Statistical analysis
All the results are presented as the mean ± standard error of the mean. Differences between 2 groups were analyzed using a Student’s t-test, while differences between multiple groups were analyzed using a 1-way analysis of variance and Duncan’s test using the SPSS 22.0 package (SPSS Inc. Chicago, IL, USA).
Results
MK expression and NETosis were detected in the diseased kidneys of the DKD patients
Pathological changes in DKD were analyzed by H&E, Masson, and PAS staining. The H&E, Masson, and PAS staining revealed that compared to the control patients who had normal tubular and glomeruli architecture (see Figure 1A-1D), there was glomerular necrosis, tubular epithelial thinning, and thickened basement membranes in the kidney tissues of the DKD patients (see Figure 1A). Additionally, the degree of renal fibrosis sees Figure 1B) and the content of the PAS-positive area (see Figure 1C,1D) were significantly increased in the kidney tissues of the DKD patients. IHC was also examined, and the results showed that the level of MK was indeed higher in the DKD than the normal kidneys (see Figure 1E,1F). IF was performed to examine the expression of the NETosis biomarkers H3Cit and MPO. The results revealed the enhanced staining of H3Cit and MPO in the DKD kidneys compared to the normal kidneys (see Figure 1G). Thus, MK expression and NETosis may play an important role in the induction of nephropathy in DKD patients.

Isolation and identification of neutrophils
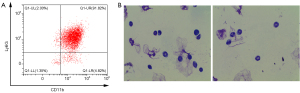
We first isolated neutrophils for the in-vitro experiments. The neutrophils were separated from the bone marrow of healthy male mice. The neutrophils were defined as CD11b+Ly6G+ cells by flow cytometry (see Figure 2A). The neutrophils were then identified by Wright staining under microscope (100× oil lenses). The microscopic images were typical of neutrophils; the horseshoe-shaped nucleus was stained blue-purple, and the cytoplasm remained light red (see Figure 2B).
Toxic effect of MK on neutrophils
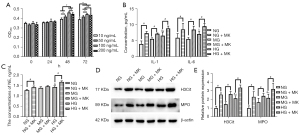
To examine the effect of MK on neutrophils, the cell viability of neutrophils treated with different concentrations of MK (10, 50, 100, and 200 ng/mL) at different durations (0, 24, 48, and 72 h) was examined by CCK-8 assays. As Figure 3A, shows there were no significant differences in the cell viability of the neutrophils treated with 10, 50, 100, and 200 ng/mL MK at 0 and 24 h (see Figure 3A); however, at 48 and 72 h, the cell viability of the neutrophils treated with 100 and 200 ng/mL of MK was significantly higher than those treated with 10 and 50 ng/mL of MK (see Figure 3A). Further, the cell viability of the neutrophils treated with 100 ng/mL of MK was the highest among the concentrations of MK at all 4-time points (see Figure 3A). Thus, we optimized the effective treatment concentration of MK and the duration at 100 ng/mL for 48 h.
MK increased neutrophil secretion of the inflammatory cytokines in vitro
In comparison to the results for the supernatant of the neutrophils cultured in conditions of NG or mannitol, the levels of neutrophil secretion of IL-1 and IL-6 was notably increased when the cells were cultured in the presence of high glucose medium (25 mM of D-glucose; see Figure 3B). Notably, MK treatment further elevated the levels of both IL-1 and IL-6 under the normal-glucose, mannitol, and high-glucose conditions (see Figure 3B). Thus, MK accelerated inflammatory cytokine secretion from neutrophils.
MK promoted NETosis in vitro
The effect of MK on neutrophil NETosis was assessed by ELISA assays. The results showed that MK treatment prominently increased the level of NE in the high glucose-induced neutrophil group compared to the NG and mannitol groups (see Figure 3C). Additionally, the Western blot results showed that MK notably elevated H3Cit and MPO protein levels under the normal-glucose, mannitol, and high-glucose culture conditions, respectively (see Figure 3D,3E). These data demonstrated that MK promoted the NETosis of neutrophils in vitro.
MK caused kidney injury in the DKD model
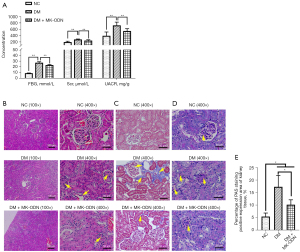
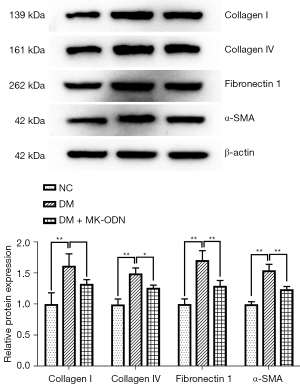
We examined the role of MK in diabetic kidney damage in vivo, using MK antisense ODN to counteract the expression of MK in the mouse DKD model. The ELISA analysis of the kidney function indexes showed that the levels of FBG, Scr, and UACR were all increased in the serum of the diabetic mice, which was significantly reduced by MK antisense ODN treatment (see Figure 4A). The H&E staining showed that the kidney tissues of the diabetic mice had a blurred glomerular structure in the cortex area, a swollen glomerular, and a thickened basement membrane, and serous-fibrous exudate was observed in the lumen of some renal tubules. The MK antisense ODN treatment significantly reduced these pathological changes in the kidney tissues of the diabetic mice (see Figure 4B). Kidney fibrosis was assessed by Masson staining. As Figure 4C shows, severe fibrosis was observed in the kidneys of the diabetic mice as assessed by Masson staining (see Figure 4C), which was significantly ameliorated by the MK antisense ODN treatment. Similarly, the PAS staining showed a significantly increased PAS stain positive area in the kidney tissues of the diabetic mice, while the MK antisense ODN treated mice showed a prominent decrease of PAS staining compared to the non-treated diabetic mice (see Figure 4D,4E). Further, the western blot results showed that the levels of fibrosis-associated proteins α-SMA, Collagen I, Collagen IV, and FN1 were all increased in the kidneys of the diabetic mice, but were all reduced by the MK antisense ODN treatment (see Figure 5). All these results demonstrated that MK caused kidney injury in diabetic mice.
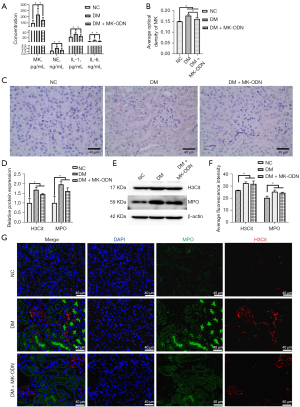
MK inhibited inflammatory factor secretion and the formation of NETosis in vivo
To further confirm the effect of MK on NETosis and the inflammatory cytokine secretion, the serum levels of IL-1 and IL-6 were analyzed in an in-vivo experiment in 3 mouse experiment groups; that is, the NC, DM, and DM + MK – ODN groups. The results showed that the serum levels of IL-1 and IL-6 were significantly increased but were reduced by the MK antisense ODN treatment in diabetic mice (see Figure 6A). Additionally, the serum levels of MK and NE were dramatically increased but were also significantly decreased by the MK antisense ODN treatment in diabetic mice (see Figure 6A). Moreover, the IHC results showed that the average optical density of MK was consistent with the ELISA results (see Figure 6B,6C). The protein levels of H3Cit and MPO were elevated in diabetic, mice but were reduced by the MK antisense ODN treatment (see Figure 6D,6E). Similarly, the IF results also showed an increase of NETosis in the diabetic mice, which was also significantly reduced by the MK antisense ODN treatment (see Figure 6F,6G). All these results showed that MK stimulated inflammation and NETosis in the kidney tissues of the diabetic mice.
Discussion
DKD is the main complication of diabetes (2). It is extremely important to elucidate the molecular mechanism and the pathogenesis of DKD to identify the key target for both the diagnosis and treatment of DKD. In this study, we found that MK expression and NETosis were increased in the kidney tissues of DKD patients. Our study proved that MK caused kidney functional and histological injuries in DKD patients by promoting NETs.
Neutrophils are the main innate immune cells of the body’s inflammatory response (30). However, the long-term persistence of neutrophil infiltration can cause tissue damage, which is closely related to the occurrence and development of many human diseases, such as diabetes mellitus (DM) and its complications (31). Previous study implied that diabetes-induced microvascular complications delays wound healing by activating NETosis in Padi4−/− mice (15). Meanwhile, NETosis is considered to be associated with the development of human diabetic foot ulcers. NETosis promoted NLRP3 inflammasome activation and IL-1β-dependent exacerbation of inflammation in diabetic foot ulcers (32). The production of NETs is involved in cytokine-induced ocular inflammation in diabetic retinopathy mice (33). In the present study, neutrophils isolated from bone marrow were used for the in-vitro assessment of MK on NETosis in DKD. The in-vitro experiments revealed that 25 mM of D-glucose significantly accelerated the formation of NETs as shown by the dramatic increase in the levels of NE, MPO, and H3Cit, which was consistent with the previous results that showed that 25 mM of glucose enhanced the release of NETs and the NETosis rate more than 5 mM glucose in vitro and in type 2 diabetic patients (34). In type 1 DM patients, NETosis formation was significantly elevated, and may serve as a risk marker for diabetes-induced renal complications (15). Furthermore, we found that the formation of NETs was enhanced in kidney tissues in human and mice DKD samples, implying that NETs may play an important role in the DKD progression. Further study is needed to better elucidate the mechanism of NETs in DKD.
NE, MPO, and H3Cit are indispensable for the formation of NETs. NE can trigger histone degradation thereby causing DNA decondensation, but can also induce actin filament degradation, thus suppressing neutrophil movement (14). MPO can also migrate to the nucleus to help NE to degrade chromatin (14). In our study, MK dramatically boosted the formation of NETs under high-glucose conditions both in vitro and in vivo, based on the increased expression of NE, MPO, and H3Cit. It has been shown that MK induced neutrophil migration and NETosis formation in the heart tissue of myocarditis patients and mouse models (20). Kosugi et al. found that the expression of MK was significantly upregulated in tubule epithelial cells and mesangial cells stimulated by high glucose in vitro, and the expression of MK was detected in glomeruli, tubules and interstitium in the kidney biopsy specimens of DKD patients (23,24). In this study, we demonstrated that MK was significantly enhanced in the kidneys of DKD patients and diabetic mice, which was reversed by the knockdown of MK via the injection of MK antisense ODN. The systemic administration of MK antisense ODN has been reported to be absorbed in the proximal tubules (29). In line with previous studies, the dosage of MK antisense ODN employed in this study was 1 mg/kg, which produced the best and most effective effects with no obvious adverse effects in murine (29,35). It is also much lower than 10 mg/kg, which indicates its good tolerance in clinical trials (36,37).
MK has also been demonstrated to play an important role in the inflammatory process of DKD (23). It was consistently observed in this study that MK significantly increases the level of IL-1 and IL-6 in vitro and in vivo, which indicates that MK might act as a direct activator of kidney inflammation in DKD (23,24). Further, in the DKD mice, MK increased Scr and UACR levels and promoted kidney injury, which is in line with results of a study using Mdk−/− mice (24). Several other studies have also revealed that the inhibition of MK prevented kidney damage in other conditions, including the onset of kidney disease in diabetes (23), 5/6 nephrectomy-induced hypertension (38), and ischemia-reperfusion (29,39). These results also suggest that the function of MK is closely related to the occurrence and progression of DKD.
Conclusions
In summary, MK promoted NETosis and inflammatory cytokine secretion in both the in-vivo and in-vitro experiments in the current study. The suppression of MK using the MK antisense ODN significantly improved kidney function and reduced pathological damage in the DKD model. In brief, the results provided a molecular mechanism for the development inhibition of MK as a clinical treatment for DKD.
Acknowledgments
Funding: This Research Project was supported by the Natural Science Foundation of Shanxi Province (No. 201901D211518), the Shanxi Scholarship Council of China (No. 2020-174), and the 136 Special Fund of Shanxi Provincial People’s Hospital (No. sz2019017).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. available at https://atm.amegroups.com/article/view/10.21037/atm-22-2382/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2382/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2382/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ethics committee of Shanxi Provincial People’s Hospital (Taiyuan, China) granted approval for the experiment procedure (No. 2022-58). All the patients provided written informed consent for this study. Animal experiments were carried out following a protocol approved by the Animal Ethics Committee of Shanxi Bethune Hospital (No. SBQKL-2021-028), in compliance with Shanxi Bethune Hospital guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 2022;183:109119. [Crossref] [PubMed]
- Schechter M, Leibowitz G, Mosenzon O. Paving the way to precision medicine for diabetic kidney disease: the PRIORITY trial. Ann Transl Med 2020;8:1698. [Crossref] [PubMed]
- Yamazaki T, Mimura I, Tanaka T, et al. Treatment of Diabetic Kidney Disease: Current and Future. Diabetes Metab J 2021;45:11-26. [Crossref] [PubMed]
- Pugliese G. Updating the natural history of diabetic nephropathy. Acta Diabetol 2014;51:905-15. [Crossref] [PubMed]
- Zhang L, Long J, Jiang W, et al. Trends in Chronic Kidney Disease in China. N Engl J Med 2016;375:905-6. [Crossref] [PubMed]
- Furuichi K, Shimizu M, Okada H, et al. Clinico-pathological features of kidney disease in diabetic cases. Clin Exp Nephrol 2018;22:1046-51. [Crossref] [PubMed]
- Chen C, Wang C, Hu C, et al. Normoalbuminuric Diabetic Kidney Disease. Front Med 2017;11:310-8. [Crossref] [PubMed]
- Fan Y, Yi Z, D'Agati VD, et al. Comparison of Kidney Transcriptomic Profiles of Early and Advanced Diabetic Nephropathy Reveals Potential New Mechanisms for Disease Progression. Diabetes 2019;68:2301-14. [Crossref] [PubMed]
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol 2017;12:2032-45. [Crossref] [PubMed]
- Jung SW, Moon JY. The role of inflammation in diabetic kidney disease. Korean J Intern Med 2021;36:753-66. [Crossref] [PubMed]
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532-5. [Crossref] [PubMed]
- Mutua V, Gershwin LJ. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin Rev Allergy Immunol 2021;61:194-211. [Crossref] [PubMed]
- Vorobjeva NV. Neutrophil Extracellular Traps: New Aspects. Moscow Univ Biol Sci Bull 2020;75:173-88. [Crossref] [PubMed]
- Nakazawa D, Marschner JA, Platen L, et al. Extracellular traps in kidney disease. Kidney Int 2018;94:1087-98. [Crossref] [PubMed]
- Njeim R, Azar WS, Fares AH, et al. NETosis contributes to the pathogenesis of diabetes and its complications. J Mol Endocrinol 2020;65:R65-76. [Crossref] [PubMed]
- Keeter WC, Moriarty AK, Galkina EV. Role of neutrophils in type 2 diabetes and associated atherosclerosis. Int J Biochem Cell Biol 2021;141:106098. [Crossref] [PubMed]
- Cai YQ, Lv Y, Mo ZC, et al. Multiple pathophysiological roles of midkine in human disease. Cytokine 2020;135:155242. [Crossref] [PubMed]
- Campbell VK, Gately RP, Krishnasamy R, et al. Midkine and chronic kidney disease-associated multisystem organ dysfunctions. Nephrol Dial Transplant 2021;36:1577-84. [Crossref] [PubMed]
- Zhang ZZ, Wang G, Yin SH, et al. Midkine: A multifaceted driver of atherosclerosis. Clin Chim Acta 2021;521:251-7. [Crossref] [PubMed]
- Weckbach LT, Grabmaier U, Uhl A, et al. Midkine drives cardiac inflammation by promoting neutrophil trafficking and NETosis in myocarditis. J Exp Med 2019;216:350-68. [Crossref] [PubMed]
- Sato W, Sato Y. Midkine in nephrogenesis, hypertension and kidney diseases. Br J Pharmacol 2014;171:879-87. [Crossref] [PubMed]
- Metwalley KA, Farghaly HS, Gabri MF, et al. Midkine: Utility as a Predictor of Early Diabetic Nephropathy in Children with Type 1 Diabetes Mellitus. J Clin Res Pediatr Endocrinol 2021;13:293-9. [Crossref] [PubMed]
- Kosugi T, Yuzawa Y, Sato W, et al. Growth Factor Midkine Is Involved in the Pathogenesis of Diabetic Nephropathy. Am J Pathol 2006;168:9-19. [Crossref] [PubMed]
- Kosugi T, Yuzawa Y, Sato W, et al. Midkine Is Involved in Tubulointerstitial Inflammation Associated with Diabetic Nephropathy. Lab Invest 2007;87:903-13. [Crossref] [PubMed]
- Ubags NDJ, Suratt BT. Isolation and Characterization of Mouse Neutrophils. Methods Mol Biol 2018;1809:45-57. [Crossref] [PubMed]
- Lackner I, Weber B, Baur M, et al. Midkine Is Elevated after Multiple Trauma and Acts Directly on Human Cardiomyocytes by Altering Their Functionality and Metabolism. Front Immunol 2019;10:1920. [Crossref] [PubMed]
- Yan J, Wang C, Jin Y, et al. Catalpol ameliorates hepatic insulin resistance in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway. Pharmacol Res 2018;130:466-80. [Crossref] [PubMed]
- Gao YF, Zhang MN, Wang TX, et al. Hypoglycemic effect of D-chiro-inositol in type 2 diabetes mellitus rats through the PI3K/Akt signaling pathway. Mol Cell Endocrinol 2016;433:26-34. [Crossref] [PubMed]
- Sato W, Takei Y, Yuzawa Y, et al. Midkine antisense oligodeoxyribonucleotide inhibits renal damage induced by ischemic reperfusion. Kidney Int 2005;67:1330-9. [Crossref] [PubMed]
- Guan H, Xie L, Ji Z, et al. Triptolide inhibits neutrophil extracellular trap formation. Ann Transl Med 2021;9:1384. [Crossref] [PubMed]
- Patel AA, Ginhoux F, Yona S. Monocytes, Macrophages, Dendritic Cells and Neutrophils: An Update on Lifespan Kinetics in Health and Disease. Immunology 2021;163:250-61. [Crossref] [PubMed]
- Lee MKS, Sreejit G, Nagareddy PR, et al. Attack of the Nets! Netosis Primes Il-1β-Mediated Inflammation in Diabetic Foot Ulcers. Clin Sci (Lond) 2020;134:1399-401. [Crossref] [PubMed]
- Barliya T, Dardik R, Nisgav Y, et al. Possible Involvement of Netosis in Inflammatory Processes in the Eye: Evidence from a Small Cohort of Patients. Mol Vis 2017;23:922-32. [PubMed]
- Menegazzo L, Ciciliot S, Poncina N, et al. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol 2015;52:497-503. [Crossref] [PubMed]
- Takei Y, Kadomatsu K, Matsuo S, et al. Antisense oligodeoxynucleotide targeted to Midkine, a heparin-binding growth factor, suppresses tumorigenicity of mouse rectal carcinoma cells. Cancer Res 2001;61:8486-91. [PubMed]
- Gheibi-Hayat SM, Jamialahmadi K. Antisense Oligonucleotide (AS-ODN) Technology: Principle, Mechanism and Challenges. Biotechnol Appl Biochem 2021;68:1086-94. [Crossref] [PubMed]
- Gagliardi M, Ashizawa AT. The Challenges and Strategies of Antisense Oligonucleotide Drug Delivery. Biomedicines 2021;9:433. [Crossref] [PubMed]
- Hobo A, Yuzawa Y, Kosugi T, et al. The growth factor midkine regulates the renin-angiotensin system in mice. J Clin Invest 2009;119:1616-25. [Crossref] [PubMed]
- Sato W, Kadomatsu K, Yuzawa Y, et al. Midkine is involved in neutrophil infiltration into the tubulointerstitium in ischemic renal injury. J Immunol 2001;167:3463-9. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


