
Systemic glucocorticoid-free therapy with adalimumab plus immunosuppressants versus conventional therapy in treatment-naïve Vogt-Koyanagi-Harada disease patients
Introduction
Vogt-Koyanagi-Harada (VKH) disease is an immune-mediated multisystem disease defined as bilateral granulomatous panuveitis with or without extraocular organ involvement, including neurological, auditory, and integumentary symptoms. Research has shown that pigmented people (e.g., Asian, Hispanic, and Native American ethnicities) are the primary targets races (1). Patients with VKH experience severe vision loss caused directly by ocular inflammation attacks or indirectly by ocular complications, ranging from cataracts, glaucoma, and choroidal neovascular membranes, which can lead to the development of subretinal fibrosis. If no proper treatment is administered, patients inevitably become blind.
The prognosis of VKH relies largely on the rapid resolution of inflammation (2). In general, ophthalmologists prefer high-dose glucocorticoids with or without immunosuppressants in the early stage of disease, and a relatively optimistic prognosis can be achieved administering this therapy. However, some issues need to be addressed. Most frequently, patients with VKH require a long-term treatment of systemic glucocorticoids. However, variable side effects emerge in up to 90% of patients who receive glucocorticoids for over 2 months regardless of the dose (3-5). Long-term systemic glucocorticoid use can result in a wide spectrum of side effects and affect multiple systems, including the endocrine and metabolic, neuropsychiatric, gastrointestinal, musculoskeletal, cardiovascular, dermatologic, ocular, and immunologic systems (5). Additionally, in glucocorticoid-resistant patients, the efficacy of glucocorticoids is limited (6). Consequently, a glucocorticoid-free therapy is needed in some special cases.
In recent years, glucocorticoid-free therapy with biologic agents was introduced into the treatment of VKH, including anti-tumor necrosis factor alpha (TNF-α) agents (infliximab) (7-9), anti-CD20 agents (rituximab) (10,11) and interferon (12). However, these cases were applied in a very limited scale and the efficacy remained debatable. Most importantly, all these cases study were focusing on refractory VKH.
Research has shown that the level of TNF-α is elevated in patients who suffer from non-infectious uveitis, including VKH. Neutralizing TNF-α with TNF-α inhibitors has been shown to be a feasible tactic in the treatment of VKH in several case reports (9,13-16). Adalimumab (ADA, Humira, AbbVie, North Chicago, Illinois, USA), a fully humanized monoclonal antibody that specifically targets TNF-α, was shown to be an ideal glucocorticoid-sparing agent in a series of rheumatic diseases (17,18). In 2016, ADA was approved for non-infectious uveitis by the Food and Drug Administration (FDA). However, to date, the few studies on ADA treatment in VKH disease have mainly targeted refractory VKH, and none of these studies have focused their attention on treatment-naïve patients who have not been systemically treated before.
Consequently, initial glucocorticoid-free therapy with ADA plus immunosuppressants in treatment-naïve VKH patients deserves further investigation. The main objective of this pilot study was to assess the feasibility, efficacy, and safety of glucocorticoid-free therapy as an initial treatment in treatment-naïve VKH patients who had an existing disease that could be exacerbated by systemic glucocorticoids. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2668/rc).
Methods
We conducted this single-center, cohort study, comparative study of patients with new-onset VKH disease who had never been systemically treated. Since this study was a cohort study, RCT was not appliable in this study. All patients received approximately 12 months (and no less than 10 months) of treatment and recommended visit monthly. This study was designed and conducted following the principles of the Helsinki Declaration (as revised in 2013). Written consent was obtained from all the participants, and the Zhongshan Ophthalmic Center Ethics Committee approved this study (No. 2020KYPJ104), and was registered in Chinese Clinical Trial Registry Center with registration No. ChiCTR200003023.
To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) have bilateral uveitis with the appearance of diffuse choroiditis and retinal exudative detachment; (II) have no previous systemic medical history of using systemic glucocorticoids or immunosuppressive agents; (III) have active ocular inflammation [anterior chamber cell/vitreous haze ≥1+ cells, optic nerve inflammation (ONI), vasculitis, retinitis, or choroiditis]; (IV) have T-SPOT test negativity or anti-tuberculosis (TB) treatments if they had tested positive in the T-SPOT test; (V) have no evidence of active infection or malignancy; (VI) have received treatment for approximately 12 months and no less than 10 months; and (VII) have refractive media that were relatively transparent and showed clear images from ocular examinations.
Patients were excluded from the study if they met any of the following exclusion criteria: (I) had a coexisting active infection (e.g., a recent immunodeficiency virus or hepatitis); (II) had a refractive medium that was too opaque to identify a clear image from fluorescein angiography (FA) and optic coherent tomography (OCT), or other ocular examinations; (III) had a history of malignant diseases; and/or (IV) had undergone any surgical procedure in the treatment period that potentially could have changed their visual function, such as cataract extraction or refractive surgeries.
Currently, it is thought that high-dose glucocorticoids are required in the early stage of VKH disease. However, in this cohort, some patients had existing systemic diseases (including gastritis, chronic enteritis, gastrointestinal ulcers, and hypertension), which are irrelevant to the ocular inflammation, but that can be exacerbated by glucocorticoids. These patients were informed of the potential risks of glucocorticoids in advance. The selection of treatment was based on a shared consensus reached between the ophthalmologists and patients. Patients were divided into the following 2 therapeutic groups according to the treatment they received: (I) the conventional therapy (CT) group, which received glucocorticoids plus immunosuppressants; and (II) the systemic glucocorticoid-free (SGF) therapy group, which received ADA plus immunosuppressants. In the CT group, 6 patients had chronic diseases, including chronic gastritis, chronic enteritis, gastrointestinal ulcers, and hypertension, or were hepatitis virus B carriers. In the SGF group, 11 patients had systemic diseases, including chronic gastritis, chronic enteritis, gastrointestinal ulcers, and hypertension. All the systemic diseases in patients in this cohort were isolated from the ocular inflammation, and were irrelevant to the onset, severity and, prognosis of the ocular disease.
Treatment: in the CT group, oral glucocorticoids were administered at 1 mg/kg/d; if severe anterior chamber inflammation or diffuse/highly serous retinal detachment (SRD) was present, methylprednisolone (MP) was administered intravenously and tapered every 3 days to 80 mg, after which oral glucocorticoids (starting at 1 mg/kg/d) was administered, and then tapered as the inflammation de-escalated. Comedication with the immunosuppressive treatments was initiated with methotrexate (MTX), cyclosporin A (CsA), or mycophenolate mofetil (MMF). Stomach protectors, potassium chloride, calcium tablets, and other supplementary agents were given to minimize the potential side effects of the glucocorticoids. Local treatments, such as glucocorticoid droplets, periocular triamcinolone acetonide (TA) and/or subconjunctival dexamethasone, were given once based on the severity of the anterior chamber inflammation and SRD. Patients were treated with prednisolone acetate (Pred Forte, Allergan, Dublin) or tobramycin dexamethasone (Tobradex, Alcon, Fort Worth, Texas, USA) for which the frequency was then decreased, and they were switched to weaker glucocorticoid droplets or withdrew as the inflammation was alleviated. Additionally, all patients received mydriatic droplets due to anterior chamber inflammation.
In the SGF group, patients initially received a loading dose of 80 mg of ADA, and 1 week later received a dose of 40 mg, followed by 40 mg every other week. Comedication with immunosuppressants and local treatments were performed using the same principle as that adopted for the CT group.
The dose of immunosuppressive agents was modified according to the symptoms and severity of VKH in all patients. Comprehensive consideration was given before the withdrawal or switching of ADA and systemic glucocorticoids, depending on the stability of the intraocular inflammation and the patients’ preferences, and other situations (e.g., an inadequate response or intolerance to certain regimens).
Outcome parameters
Intraocular inflammation (including anterior chamber cell grade and vitritis grade), best-corrected visual acuity (BCVA), central macular thickness (CMT), ONI, intraocular manifestations, and the number of relapses and adverse events (AEs) were recorded and compared at the baseline and at the last visit. Monthly BCVA and glucocorticoid dosage data are depicted in a line chart.
Visual acuity
BCVA was determined by the Snellen chart. Extremely low BCVA classified by a semiquantitative scale as “counting finger (CF)/hand motion (HM)/light perception (LP)” was converted to a quantified visual acuity value for statistical convenience (19).
Anterior chamber cell grade and vitritis grade
The anterior chamber cell grade was evaluated by slit-lamp biomicroscopy and recorded according to the 1990 recommendations of the Standardization of Uveitis Nomenclature (SUN) Working Group (20). The degree of vitreous haze was evaluated by the Nussenblatt scale (21).
CMT
CMT measurements were measured by Cirrus OCT (Carl Zeiss, CA, USA). CMT was defined as the average retinal thickness within a 1 mm2 diameter region in the macular fovea.
ONI
ONI was defined as the presence of optic nerve staining in the late stage shown by FA.
Intraocular/extraocular manifestations and complications
Intraocular manifestations included SRD, sunset glow fundus, pigmentation scatter, iris nodules, subretinal fibrosis, and any other complications. Extraocular manifestations included neurological, auditory, and integumentary systems. Most of these pathognomonic findings implied that the disease course had progressed to the chronic recurrent stage with the exception of SRD. Cataracts, intraocular pressure >21 mmHg (or even glaucoma), band-shaped keratopathy, and any other complications were also recorded.
Glucocorticoid dosage
Since systemic glucocorticoids were initially used only in the CT group, the observation of monthly variation in glucocorticoids was mainly applied in the CT group. However, if patients in the SGF group switched to glucocorticoids or received additional glucocorticoids, the glucocorticoid dosages were recorded. Topical glucocorticoids, such as subconjunctival or periocular glucocorticoids, were not included in the systemic glucocorticoid dosage.
Relapse
Relapse was defined as the renewed presence (from inactive inflammation to active inflammation as per the definition of Standardization of the Uveitis Nomenclature Group) or the exacerbation of ocular inflammation (20,22).
System disorder monitoring
General symptoms of VKH (including neurological, auditory, and integumentary symptoms), routine blood examinations, liver and renal function, and electrocardiography were monitored during treatment. Any system disorder was recorded and analyzed.
AEs
AEs included (I) recent onset infections or reinfections of latent diseases; (II) gastrointestinal disease; (III) injection reactions or allergy; and (IV) any other situations. The severe AEs were classified as per the events list by the European Medicines Agency (https://www.ema.europa.eu/en/documents/other/eudravigilance-inclusion/exclusion-criteria-important-medical-events-list_en.pdf). The severe AEs included those that can result in death, were life threatening, required hospitalization or prolonged existing hospitalization, and resulted in persistent or significant disability, or birth defects.
Statistical analysis
The continuous variables were described by the mean ± standard deviation or median [interquartile range (IQR)/range], and the categorical variables were described by the number and percentage. The baseline characters comparability was performed by t-tests, Mann-Whitney U-tests, or chi-square tests were used for comparisons between the 2 treatment groups depending on the variable type. SPSS 25.0 was used for the analysis, and a P value <0.05 (two sided) was considered statistically significant.
Results
Baseline demographics in different groups
A total of 30 consecutive patients were recruited and analyzed. Baseline profiles were comparable in both groups. All of the enrolled patients were at the typical early stage of the VKH course according to the revised diagnostic criteria for VKH disease (i.e., had the appearance of diffuse choroiditis and retinal exudative detachment) (23). Of these patients, 19 patients (63.33%) were treated with CT, and 11 (36.67%) were treated with SGF as the initial treatment. In both groups, the sex composition was similar (6 males and 13 females in the CT group, and 3 males and 8 females in the SGF group; P=1.000). The mean age in both groups did not differ significantly {40 [28, 49] years in the CT group, and 46 [40, 55] years in the SGF group; P=0.059}. The previous history from onset to presentation at our outpatient department was 0 (0, 2) weeks in the CT group, and 0 (0, 2) weeks in the SGF group (P=0.693). In relation to the classification of the disease, the groups were differently constituted; that is, in the CT group, 6 patients had complete VKH disease, 8 had incomplete VKH disease, and 5 had probable VKH disease, and in the SGF group, 6 patients had complete VKH disease, and 5 had incomplete VKH disease (P=0.213). Both groups received approximately 12 months of treatment {12 [11, 13] months in the CT group, and 13 [10, 13] months in the SGF group; P=0.812}. The longest follow-up time was 15 months and the shortest was 10 months in both groups. Patients’ demographic characteristics are presented in Table 1. No patient underwent any surgical procedure in the treatment period.
Table 1
| Group | CT (n=19) | SGF (n=11) | P |
|---|---|---|---|
| Patients/eyes | 19/38 | 11/22 | – |
| Age, median [IQR] years | 40 [28, 49] | 46 [40, 55] | 0.059 |
| Sex, male/female | 6/13 | 3/8 | 1.000 |
| Classification (complete/incomplete/probable) | 6/8/5 | 6/5/0 | 0.213 |
| Duration of the previous disease course, median [IQR] weeks | 0 [0, 2] | 0 [0, 2] | 0.693 |
| Treatment period, median [IQR] months | 12 [11, 13] | 13 [10, 13] | 0.812 |
Mann-Whitney U-tests were used to compare age, previous disease course, and treatment period; Chi-square tests were used to compare sex and classifications. P<0.05 was considered as statistically significant. VKH, Vogt-Koyanagi-Harada; CT, conventional therapy; SGF, systemic glucocorticoid-free therapy; IQR, interquartile range.
Ocular parameters at the baseline in different groups
The ocular parameters were comparable at the baseline in the 2 groups and are presented in Table 2. BCVA (0.28±0.22 in the CT group, 0.22±0.19 in the SGF group, P=0.290) and CMT (622.11±412.79 µm in the CT group, 566.32±397.11 µm in the SGF group, P=0.605) were similar at the baseline. The anterior chamber cell grade was approximately the same in both groups {2 [1, 2] in the CT group, 2 [1, 2] in the SGF group; P=0.262}, as was the vitritis grade {1 [0, 1.25] in the CT group, 1 [1, 1] in the SGF group; P=0.188}. Since all patients were at a typical early stage, SRD and ONI were found in all 30 patients from the 2 groups. Other complications, such as sunset glow fundus, Dalen-Fuchs nodules, pigmentation scatter, iris nodules, and subretinal fibrosis, were not observed in any patients in this study before the treatment commenced (see Figure 1).
Table 2
| Group | Baseline | Changes at the final visit | |||||
|---|---|---|---|---|---|---|---|
| CT (n=38) | SGF (n=22) | P | CT (n=38) | SGF (n=22) | P | ||
| BCVA, mean± SD | 0.28±0.22 | 0.22±0.19 | 0.290 | 0.40±0.26 | 0.57±0.23 | 0.014* | |
| AC cell grade, median (IQR) | 2 (1, 2) | 2 (1, 2) | 0.262 | –1.5 (–2, –0.5) | –1 (–2, –1) | 0.367 | |
| Vitritis grade, median (IQR) | 1 (0, 1.25) | 1 (1, 1) | 0.188 | 0 (–1.25, 0) | –1 (–1, –1) | 0.050 | |
| CMT, mean ± SD μm | 622.11±412.79 | 566.32±379.11 | 0.605 | –423.47±412.09 | –362.73±375.73 | 0.572 | |
| Relapse, times, median (IQR) | – | – | – | 1 (0, 2) | 0 (0, 2) | 0.372 | |
Changes in clinical features are posttreatment values subtracted from their pretreatment counterparts. Independent sample t-tests were used to compare BCVA and CMT; Mann-Whitney U-tests were used to compare AC cell, vitritis, and relapses. *, P<0.05. VKH, Vogt-Koyanagi-Harada; AC, anterior chamber; BCVA, best-corrected visual acuity; CT, conventional therapy; SGF, systemic glucocorticoid-free therapy; CMT, central macular thickness.
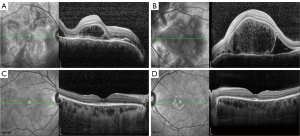
Therapeutic management of different groups
In the CT group, 14 of the 19 patients (73.68%) initially received intravenous methylprednisolone, with an average dosage of 177.14±60.18 mg/d. Oral prednisone was initiated to all patients from the CT group, with an average dosage of 60.00±4.41 mg/d. In the SGF group, no patient initially received any form of systemic glucocorticoid except for a topical glucocorticoid once (via a subconjunctival injection of dexamethasone and/or a periocular injection of TA). No significant difference was found between the groups in relation to the prescription of the immunosuppressive agents (see Table 3). Local treatments were similar in both groups.
Table 3
| Group | CT (n=19) | SGF (n=11) | P |
|---|---|---|---|
| Systemic treatments at baseline, patients | |||
| IV methylprednisolone | 14 (73.68%) | 0 | <0.001* |
| Oral glucocorticoid | 19 (100.00%) | 0 | <0.001* |
| MTX | 18 (94.74%) | 10 (90.91%) | 1.000 |
| CsA | 6 (31.58%) | 9 (81.82%) | 0.021* |
| MMF | 0 | 1 (9.09%) | 0.367 |
| CTX | 1 (5.26%) | 0 | 1.000 |
| Othersa | 1 (5.26%) | 0 | 1.000 |
| Dosage at baseline, mean ± SD | |||
| IV methylprednisolone, mg qd | 177.14±60.18 | – | – |
| Oral glucocorticoid, mg qd | 60.00±4.41 | – | – |
| MTX, mg qw | 12.64±2.5 | 15.00±0.00 | 0.001* |
| CsA, mg bid | 87.50±13.69 | 50.00±0.00 | 0.001* |
| MMF, g bid | – | 0.25 | – |
| CTX, g qd | 0.4 | – | – |
| Systemic treatments at the final visit, patients | |||
| Oral glucocorticoid | 8 (42.11%) | 0 | 0.014* |
| MTX | 14 (73.68%) | 8 (72.73%) | 1.000 |
| CsA | 4 (21.5%) | 3 (27.27%) | 1.000 |
| MMF | 3 (15.79%) | 1 (9.09%) | 1.000 |
| Dosage at the final visit, mean ± SD | |||
| Oral glucocorticoid, mg qd | 10.00±5.35 | – | – |
| MTX, mg qw | 11.43±3.63 | 11.25±2.32 | 0.902 |
| CsA, mg bid | 50.00±20.41 | 33.33±14.43 | 0.286 |
| MMF, g bid | 0.42±0.14 | 0.50 | 0.667 |
Othersa: Thalidomide was administered to 1 patient in the conventional treatment group. Independent sample t-tests were used to compare medication dosages; chi-square tests were used to compare medication categories. CT, conventional therapy; IV, intravenous injection; CsA, cyclosporin A; MTX, methotrexate; CTX, cyclophosphamide; SGF, systemic glucocorticoid-free therapy; MMF, mycophenolate mofetil. *, P<0.05.
Ocular parameter improvement in the different groups at the last visit
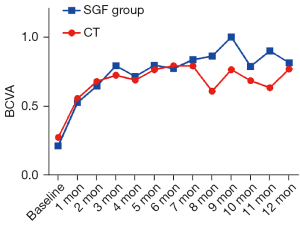
BCVA was obviously improved in both groups during the first 2 months of treatment, and the BCVA improvements were slightly better in the SGF group than the CT group (BCVA improvement in the CT group: 0.40±0.26, in the SGF group: –0.57±0.23; P=0.014; see Figure 2).
Decreases in the anterior chamber cell grade and vitritis grade were similar in both groups; the anterior chamber cell improved by a grade of –1.5 (–2, –0.5) in the CT group and –1 (–2, –1) in the SGF group (P=0.367), and vitritis improved by a grade of 0 (–1.25, 0) in the CT group and –1 (–1, –1) in the SGF group (P=0.050). The decrease in CMT was similar in the CT group compared to the SGF group (–423.47±412.09 µm in the CT group, –362.73±375.73 µm in the SGF group; P=0.572). Detailed information is provided in Table 2.
Ocular complications were found. Specifically, SRD remained in 1 patient (1 eye, 2.63%) in the CT group due to secondary central serous chorioretinopathy. The temporary elevation of intraocular pressure >21 mmHg was observed in 6 patients (7 eyes, 18.42%) in the CT group and 1 patient (2 eyes, 5.26%) in the SGF group and was quickly controlled with timorium droplets/brinzolamide and timolol maleate droplets or even simply by observation without intervention. Sunset flow fundus was observed in 11 patients (22 eyes, 57.89%) in the CT group and 3 patients (6 eyes, 27.27%) in the SGF group (P=0.142). ONI had disappeared in all patients by the final visit. Subretinal fibrosis was observed in 2 patients (4 eyes, 10.53%) in the CT group, but was not observed in any patients in the SGF group. During the treatment period, iris nodules were found in 2 patients (4 eyes, 10.53%) in the CT group but not in any patients in the SGF group. During the treatment period, pigmentation scatter was found in 3 patients (6 eyes, 15.79%) in the CT group but not in any patients in the SGF group. The new onset of mild complicated cataracts was found in 5 patients (10 eyes, 26.32%) in the CT group and 4 patients (6 eyes, 27.27%) in the SGF group.
Relapses, glucocorticoid dosage, strategy switching, system disorder monitoring, extraocular manifestations and AEs in the different groups
In the CT group, the median number of relapses was 1 (0, 2), and in the SGF group, the median number of relapses was 0 (0, 2) (P=0.372). The 2 patients who suffered from relapse in the SGF group discontinued ADA and other medications for nearly 2 months due to the inaccessibility of ADA during the Coronavirus Disease 2019 epidemic. All the patients in the CT group initially received oral prednisone at an average dosage of 60.0±4.41 mg. In the SGF group, patients received an average of 13.55±5.16 injections of ADA.
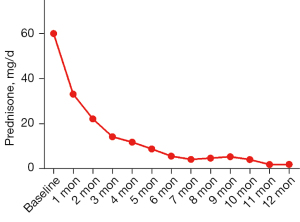
After approximately 12 months of treatment, an average dosage of 10.00±5.35 mg/d prednisone was used in the remaining 8 patients in the CT group (see Figure 3). In the CT group, 1 patient switched to ADA treatment, and 1 patient received tofacitinib (Xelijanz, Pfizer, New York, USA) due to the limited control of the inflammation. In the SGF group, 7 of the 11 patients withdrew from ADA under the guidance of ophthalmologists, 1 patient suspended ADA due to the inaccessibility of ADA during the Corona Virus Disease 2019 (COVID-19) pandemic, and 3 patients are presumed to have received ADA treatment. In the SGF group, 1 patient switched to CT due to lumbar herpes zoster.
The general symptoms of VKH disappeared or were substantially alleviated in both groups. In the CT group, all the general symptoms of the patients disappeared, except for 1 patient who had a headache and 1 patient who had long-term tinnitus after withdrawing from the glucocorticoids. In the SGF group, all the general symptoms of the patients disappeared, except for 2 patients who had interim and mild tinnitus, and 3 patients who suffered from headache/dizziness.
In relation to the AEs, in the CT group, no severe AEs were reported; however, 4 patients complained of joint pain or muscle pain, 1 patient had a skin rash, 1 patient had diarrhea, and 2 patients had vomiting. In the SGF group, no severe AEs were reported; however, 3 patients had mild local reactions at the injection site that disappeared after ice cooling, and 1 patient had diarrhea. In the SGF group, 1 patient switched to CT due to lumbar herpes zoster and soon recovered after receiving an anti-virus treatment. No patient discontinued SGF treatment due to severe AEs. No severe abnormities in the serological tests (including routine blood examination, urological examination, coagulation function, erythrocyte sedimentation rate, blood glucose, and hepatorenal function) and electrocardiograms were found.
Discussion
With an incidence rate of 15.9%, VKH is one of the most common entities of uveitis in Chinese patients (24). Somewhat concerningly, in addition to the high incidence of VKH, even if patients receive timely treatment, approximately half of the patients retain a normal fundus, while another half show late phase signs (e.g., sunset glow fundus and pigmentation scatter) (2). If early and proper treatment is not administered, VKH tends to progress to recurrent anterior uveitis, and negative sequelae, such as complicated cataracts, secondary glaucoma, subretinal fibrosis, and band-shaped degeneration of the cornea, occurs in approximately 50% of VKH patients (25-27). Ultimately, patients suffer from severe and permanent visual impairment.
Previous studies have shown that the rapid resolution of intraocular inflammation in the early stage and the restoration of retinal morphology result in fewer recurrences and depigmentation (28,29). Unfortunately, there is no consensus as to the standard therapy for VKH patients. Currently, the mainstay of treatment in VKH is high-dose intensive systemic glucocorticoids with or without immunosuppressive agents. For intensive treatment, up to 1 mg/kg/d of glucocorticoids is frequently administered for at least 6 months (30-32), followed by a long-term prolonged maintenance-dose glucocorticoid treatment; however, this is a double-edged sword, as it is accompanied by various side effects (e.g., cardiovascular disease, obesity, infection, hyperglycemia, adrenal suppression, Cushingoid phenotype, osteoporosis, cataracts, and glaucoma) (3-5). Even if an optimized therapeutic regimen of conventional therapy that aims to reduce the dosage of glucocorticoid is implemented, the aforementioned side effects are inevitable, and sunset glow fundus and pigmentation scatter newly appear in 22.9% and 23.8% of patients, respectively (2). Another limitation of conventional glucocorticoid treatment in inflammatory diseases is glucocorticoid-resistance (6,33). Thus, an alternative therapy of conventional systemic glucocorticoid treatment is needed in some special cases.
In recent years, TNF-α inhibitors have been proven to be a feasible option for the treatment of non-infective uveitis, including ADA. In 2013, an expert recommendation indicated that ADA should be regarded as a 2nd-line medication for severe posterior uveitis and panuveitis refractory to glucocorticoids (34). In our published article and submitted manuscripts on Behcet’s uveitis, ADA effectively alleviated the inflammation and reduced the daily glucocorticoid dosage (35,36). However, the application of TNF-α inhibitors in VKH has only been reported on a limited scale. Díaz-Llopis et al. and Nguyen et al. conducted multicenter studies on the efficacy of ADA in treating sight-threatening non-infectious uveitis, including VKH, in a few patients, and concluded that ADA was effective in anti-inflammatory activity and reduced the dose of glucocorticoid required (37,38). However, these studies did not mention the details of the VKH patients, and it remains unclear whether ADA was effective in subgroups of VKH patients. Additionally, a few studies have specifically emphasized the efficacy of ADA in treating VKH patients who were refractory to the combotherapy of systemic glucocorticoid and immunosuppressive agents, and have revealed similar outcomes; that is, that ADA is effective, safe, and helpful in reducing the dose of oral glucocorticoids (15,39). However, these studies were case reports, and all of these studies focused on the efficacy of ADA in refractory VKH instead of as an initial treatment in naïve-VKH patients. Most importantly, the patients in these studies had received systemic glucocorticoids, and thus there was no evidence of the feasibility of adopting a glucocorticoid-free strategy in treating VKH patients.
In this study, despite the potential interference of discontinuing treatment due to quarantine, we found a number of benefits in applying SGF therapy at an early stage of VKH. Notably, the substantial benefits related to visual outcomes, ocular inflammation, relapse frequency, macular morphology, and intraocular manifestations, and did not carry any affiliated severe risks. The efficacy of the SGF therapy was roughly comparable to that of CT across multiple dimensions. However, it should be emphasized again that the CT of glucocorticoids plus immunosuppressants is effective for most patients if administered properly, and SGF therapy was originally designed as a supplement for some special cases. Additionally, SGF did not mean that the treatment was completely glucocorticoid-free, and topical glucocorticoids were administered in this study.
The efficacy of isolated subtenon TA injections in the treatment of acute VKH disease in a small group of patients has been discussed (40). Of the 9 patients diagnosed with VKH disease without general symptoms, the subtenon injections of TA were effective. However, in the 5 patients with general symptoms, the TA injections failed to control either the ocular or general symptoms of 3 of the patients. Conversely, for most patients (14 of 19 patients in the CT group and all 11 patients in the SGF group) in our cohort with severe general symptoms, subtenon injections of TA alone represented a potentially risky option. Second, due to a number of issues in this study, including the small sample size, the application of systemic glucocorticoids to some patients, and the repeat injections, isolated subtenon TA injections were an inappropriate option for this cohort of patients. As most patients in this study had severe SRD at the baseline, a single injection of TA was applied as a reinforcement of the systemic treatment in both groups. This pilot study merely intended to prove the feasibility of SGF therapy as an alternative of CT, but the question of whether SGF therapy is better than CT and absolute glucocorticoid-free therapy without topical glucocorticoids requires further research due to the relatively small sample size of this study.
Over time, it has been reported that there is a minor possibility of AEs, including cancer, demyelinating disease, opportunistic infections, tuberculosis, and cardiovascular diseases, in patients who receive ADA treatment (41-43). In this study, no severe AEs were observed during the treatment; however, this may have been due to the limited observation period and the relatively small patient cohort. Thus, more patients and a longer observation period are required to assess the long-term risk of severe AEs.
This appears to be the first study to prove the feasibility of SGF therapy in the treatment of treatment-naïve VKH patients. Additionally, this appears to be the first study to explore the efficacy of ADA in treatment-naïve VKH patients. However, limitations were unavoidable. As this was a pilot study, the patient scale of the SGF group was limited. Second, the optimal timing of ADA withdrawal remains unknown. According to previous research, the premature withdrawal of ADA can result in recurrence (39). However, the prolonged injection of ADA raises the possibility of forming antidrug antibodies against ADA (44-47). Thus, further studies need to be conducted.
Conclusions
SGF therapy is feasible for treatment-naïve VKH patients. SGF therapy is effective, safe, and well-tolerated, which makes it a feasible option in patients with patients had existing systemic diseases.
Acknowledgments
Funding: This work was supported by the National Key Research and Development Program of China (No. 2017YFA0105804), and the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (No. 2017BT01S138).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2668/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2668/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2668/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was designed and conducted following the principles of the Helsinki Declaration (as revised in 2013). Ethics approval was granted from the Ethics Committee of Zhongshan Ophthalmic Center (No. 2020KYPJ104), and was registered in Chinese Clinical Trial Registry Center with registration No. ChiCTR200003023. Written informed consent was obtained from all the participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Du L, Kijlstra A, Yang P. Vogt-Koyanagi-Harada disease: Novel insights into pathophysiology, diagnosis and treatment. Prog Retin Eye Res 2016;52:84-111. [Crossref] [PubMed]
- Yang P, Ye Z, Du L, et al. Novel treatment regimen of Vogt-Koyanagi-Harada disease with a reduced dose of corticosteroids combined with immunosuppressive agents. Curr Eye Res 2018;43:254-61. [Crossref] [PubMed]
- Curtis JR, Westfall AO, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum 2006;55:420-6. [Crossref] [PubMed]
- Pereira RM, Carvalho JF, Canalis E. Glucocorticoid-induced osteoporosis in rheumatic diseases. Clinics (Sao Paulo) 2010;65:1197-205. [Crossref] [PubMed]
- Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf 2016;15:457-65. [Crossref] [PubMed]
- Vandewalle J, Luypaert A, De Bosscher K, et al. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol Metab 2018;29:42-54. [Crossref] [PubMed]
- Zmuda M, Tiev KP, Knoeri J, et al. Successful use of infliximab therapy in sight-threatening corticosteroid-resistant Vogt-Koyanagi-Harada disease. Ocul Immunol Inflamm 2013;21:310-6. [Crossref] [PubMed]
- Suhler EB, Smith JR, Giles TR, et al. Infliximab therapy for refractory uveitis: 2-year results of a prospective trial. Arch Ophthalmol 2009;127:819-22. [Crossref] [PubMed]
- Khalifa YM, Bailony MR, Acharya NR. Treatment of pediatric vogt-koyanagi-harada syndrome with infliximab. Ocul Immunol Inflamm 2010;18:218-22. [Crossref] [PubMed]
- Dolz-Marco R, Gallego-Pinazo R, Díaz-Llopis M. Rituximab in refractory Vogt-Koyanagi-Harada disease. J Ophthalmic Inflamm Infect 2011;1:177-80. [Crossref] [PubMed]
- Umran R, Shukur Z. Rituximab for sight-threatening refractory pediatric Vogt-Koyanagi-Harada disease. Mod Rheumatol 2018;28:197-9. [Crossref] [PubMed]
- Touitou V, Sene D, Fardeau C, et al. Interferon-alpha2a and Vogt-Koyanagi-Harada disease: a double-edged sword. Int Ophthalmol 2007;27:211-5. [Crossref] [PubMed]
- Niccoli L, Nannini C, Cassarà E, et al. Efficacy of infliximab therapy in two patients with refractory Vogt-Koyanagi-Harada disease. Br J Ophthalmol 2009;93:1553-4. [Crossref] [PubMed]
- Budmann GA, Franco LG, Pringe A. Long term treatment with infliximab in pediatric Vogt-Koyanagi-Harada disease. Am J Ophthalmol Case Rep 2018;11:139-41. [Crossref] [PubMed]
- Su E, Oza VS, Latkany P. A case of recalcitrant pediatric Vogt-Koyanagi-Harada disease successfully controlled with adalimumab. J Formos Med Assoc 2019;118:945-50. [Crossref] [PubMed]
- Takayama K, Obata H, Takeuchi M. Efficacy of Adalimumab for Chronic Vogt-Koyanagi-Harada Disease Refractory to Conventional Corticosteroids and Immunosuppressive Therapy and Complicated by Central Serous Chorioretinopathy. Ocul Immunol Inflamm 2020;28:509-12. [Crossref] [PubMed]
- Xu J, Qin Y, Chang R, et al. Aqueous cytokine levels in four common uveitis entities. Int Immunopharmacol 2020;78:106021. [Crossref] [PubMed]
- Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis 2013;72:517-24. [Crossref] [PubMed]
- Ramanan AV, Dick AD, Jones AP, et al. Adalimumab plus Methotrexate for Uveitis in Juvenile Idiopathic Arthritis. N Engl J Med 2017;376:1637-1646. [Crossref] [PubMed]
- Schulze-Bonsel K, Feltgen N, Burau H, et al. Visual acuities "hand motion" and "counting fingers" can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci 2006;47:1236-40. [Crossref] [PubMed]
- Jabs DA, Nussenblatt RB, Rosenbaum JT, et al. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005;140:509-16. [Crossref] [PubMed]
- Nussenblatt RB, Palestine AG, Chan CC, et al. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology 1985;92:467-71. [Crossref] [PubMed]
- Jaffe GJ, Dick AD, Brézin AP, et al. Adalimumab in Patients with Active Noninfectious Uveitis. N Engl J Med 2016;375:932-43. [Crossref] [PubMed]
- Read RW, Holland GN, Rao NA, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol 2001;131:647-52. [Crossref] [PubMed]
- Yang P, Zhang Z, Zhou H, et al. Clinical patterns and characteristics of uveitis in a tertiary center for uveitis in China. Curr Eye Res 2005;30:943-8. [Crossref] [PubMed]
- Read RW, Rechodouni A, Butani N, et al. Complications and prognostic factors in Vogt-Koyanagi-Harada disease. Am J Ophthalmol 2001;131:599-606. [Crossref] [PubMed]
- Fang W, Yang P. Vogt-koyanagi-harada syndrome. Curr Eye Res 2008;33:517-23. [Crossref] [PubMed]
- Yang P, Sun M. Band-shaped keratopathy in Chinese patients with Vogt-Koyanagi-Harada syndrome. Cornea 2011;30:1336-40. [Crossref] [PubMed]
- Chee SP, Jap A, Bacsal K. Spectrum of Vogt-Koyanagi-Harada disease in Singapore. Int Ophthalmol 2007;27:137-42. [Crossref] [PubMed]
- Miyanaga M, Kawaguchi T, Shimizu K, et al. Influence of early cerebrospinal fluid-guided diagnosis and early high-dose corticosteroid therapy on ocular outcomes of Vogt-Koyanagi-Harada disease. Int Ophthalmol 2007;27:183-8. [Crossref] [PubMed]
- Moorthy RS, Inomata H, Rao NA. Vogt-Koyanagi-Harada syndrome. Surv Ophthalmol 1995;39:265-92. [Crossref] [PubMed]
- Read RW, Yu F, Accorinti M, et al. Evaluation of the effect on outcomes of the route of administration of corticosteroids in acute Vogt-Koyanagi-Harada disease. Am J Ophthalmol 2006;142:119-24. [Crossref] [PubMed]
- Nazari H, Rao NA. Resolution of subretinal fluid with systemic corticosteroid treatment in acute Vogt-Koyanagi-Harada disease. Br J Ophthalmol 2012;96:1410-4. [Crossref] [PubMed]
- Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci 2012;1261:55-63. [Crossref] [PubMed]
- Levy-Clarke G, Jabs DA, Read RW, et al. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology 2014;121:785-96.e3. [Crossref] [PubMed]
- Hu Y, Huang Z, Yang S, et al. Effectiveness and Safety of Anti-Tumor Necrosis Factor-Alpha Agents Treatment in Behcets' Disease-Associated Uveitis: A Systematic Review and Meta-Analysis. Front Pharmacol 2020;11:941. [Crossref] [PubMed]
- Díaz-Llopis M, Salom D, Garcia-de-Vicuña C, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology 2012;119:1575-81. [Crossref] [PubMed]
- Nguyen QD, Merrill PT, Jaffe GJ, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet 2016;388:1183-92. [Crossref] [PubMed]
- Couto C, Schlaen A, Frick M, et al. Adalimumab Treatment in Patients with Vogt-Koyanagi-Harada Disease. Ocul Immunol Inflamm 2018;26:485-9. [Crossref] [PubMed]
- Hosoda Y, Hayashi H, Kuriyama S. Posterior subtenon triamcinolone acetonide injection as a primary treatment in eyes with acute Vogt-Koyanagi-Harada disease. Br J Ophthalmol 2015;99:1211-4. [Crossref] [PubMed]
- Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001;345:1098-104. [Crossref] [PubMed]
- Solomon AJ, Spain RI, Kruer MC, et al. Inflammatory neurological disease in patients treated with tumor necrosis factor alpha inhibitors. Mult Scler 2011;17:1472-87. [Crossref] [PubMed]
- Ruderman EM. Overview of safety of non-biologic and biologic DMARDs. Rheumatology (Oxford) 2012;51:vi37-43. [Crossref] [PubMed]
- van Schouwenburg PA, Rispens T, Wolbink GJ. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol 2013;9:164-72. [Crossref] [PubMed]
- Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol 2017;13:707-18. [Crossref] [PubMed]
- Strand V, Balsa A, Al-Saleh J, et al. Immunogenicity of Biologics in Chronic Inflammatory Diseases: A Systematic Review. BioDrugs 2017;31:299-316. [Crossref] [PubMed]
- Cohen SB, Alonso-Ruiz A, Klimiuk PA, et al. Similar efficacy, safety and immunogenicity of adalimumab biosimilar BI 695501 and Humira reference product in patients with moderately to severely active rheumatoid arthritis: results from the phase III randomised VOLTAIRE-RA equivalence study. Ann Rheum Dis 2018;77:914-21. [Crossref] [PubMed]


