
Anti-atherosclerotic effects of genistein in preventing ox-low-density lipoprotein-induced smooth muscle-derived foam cell formation via inhibiting SRC expression and L-Ca channel currents
Introduction
As a chronic inflammatory disorder of large and medium-sized arteries, atherosclerosis (AS) contributes to stroke, peripheral vascular disease, and ischemic heart disease, collectively named cardiovascular diseases (CVDs) (1). The profile of risks carried by low-density lipoprotein (LDL) cholesterol, blood pressure and smoking has been shifting to triglyceride-rich lipoproteins and LDL as contributors in AS (2). The oxidized low-density lipoprotein (ox-LDL) is abundant in atherosclerotic lesions showing proinflammatory and immune-stimulatory properties, which contributes to development of AS, plaque rupture, and CVDs (3). Atherosclerotic lesions are majorly characterized by lipid deposition in some arteries, accompanied by smooth muscle cell (SMC) and fibrous matrix proliferation, which gradually progresses to atherosclerotic plaque formation (4). Foam cells have been implicated in all stages of atherosclerotic lesion development, a large part of which originate from vascular SMCs (VSMCs) (5). Moreover, the rapid deposition of oxidized lipids, including ox-LDL, in the circulation results in migration and foaming necrosis of a large amount of VSMCs, thus facilitating AS formation (6,7). Of note, the involvement of L-type calcium (L-Ca) channels has been revealed in VSMC migration (8). Therefore, there is an ongoing urgent need to ascertain the molecular mechanism behind L-Ca channels and VSMC proliferation, migration, and foaming in AS for the exploration of promising targets for AS treatment.
As a biologically active isoflavone existing in soy, genistein orchestrates multiple pathophysiologic pathways linked to cancer, metabolic syndrome, and obesity (9). A prior study elaborated the atheroprotective effects of genistein in apolipoprotein E-deficient rats (10). Genistein has also been reported to decrease VSMC proliferation caused by ox-LDL (11). Of note, the study by Ono et al. manifested that genistein restrained SRC proto-oncogene, non-receptor tyrosine kinase (SRC)-induced cell proliferation in human gallbladder carcinoma (12). The SRC protein belongs to the SRC family of protein tyrosine kinases that are present in all mammalian cells and involved in numerous cell processes like differentiation, proliferation, and migration (13). The implication of SRC has been displayed in foam cell formation in macrophages and lesion development in AS (14). Furthermore, SRC could activate L-Ca channels in immature mouse cardiomyocytes (15). Meanwhile, calcium voltage-gated channel subunit alpha1 C (CACNA1C) encodes Ca1.2 of L-Ca channels, which participates in the process of AS (16,17). A study uncovered that CACNA1C was related to the upregulation of lectin-like oxidized LDL receptor-1 (LOX-1) during the differentiation and activation of macrophages (18). Notably, a recent study showed that LOX-1 was upregulated in AS rats, which accelerated ox-LDL uptake in the aorta of the rats (19). Still, the molecular causes leading to VSMC proliferation, migration, and foaming are poorly understood, and effective treatments for AS are slow to emerge. There remain many unanswered questions in regards to the downstream mechanistic actions of genistein and the involvement of L-Ca channel currents in the biological characteristics of VSMCs. These previously uncharacterized questions are considered in the present study.
In this context, we speculated that genistein might control AS progression via the SRC/CACNA1C/LOX-1 axis. Therefore, an AS rat model was established to identify SRC, CACNA1C, and LOX-1 expression after genistein treatment, and a cell model was developed on VSMCs using ox-LDL to further dissect their role in AS by measuring the proliferation, migration, and foaming of VSMCs. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2113/rc).
Methods
AS rat model establishment
A total of 56 12-week-old specific-pathogen-free (SPF) Sprague-Dawley (SD) rats were purchased from the Laboratory Animal Center, Harbin Medical University (Heilongjiang, China). Rats were individually housed in an SPF animal laboratory with 60–65% humidity at 22–25 ℃. The experimentations were started after 1-week of acclimatization. The health status of rats was observed prior to the experiments. Animal experiments were performed under a project license (No. 2021086) granted by Animal Ethical Care Committee of the First Affiliated Hospital of Harbin Medical University, in compliance with institutional guidelines for the care and use of animals. A protocol was prepared before the study without registration.
We randomly selected 8 rats from the 56 to undergo sham-operation, and fed them with conventional chow for 12 weeks. The remaining rats were fed with a high-fat diet (HFD; D12108C, Biopike, Beijing, China) containing 1.25% cholesterol, 10% fat, and 0.1% propylthiouracil for 12 weeks to induce the AS model.
The untreated AS rats served as the AS group, and the remaining AS rats were treated with dimethyl sulfoxide (DMSO), genistein, DMSO+ overexpression (oe)-negative control (NC), genistein + oe-NC, and genistein + oe-LOX-1 (8 rats/group). For the genistein treatment, rats were fed with HFD and gavaged daily with 45 mg/kg genistein or an equivalent volume of DMSO (abs47048069; Absin Bioscience Inc., Shanghai, China) as control. Besides, lentivirus-based LOX-1 overexpression vectors were constructed with a titer of 1×108 TU/mL. While being fed with the HFD, rats were injected with 0.1 mL lentiviruses via the tail vein every 3 days. After 12 weeks, rats were euthanized using the carbon dioxide asphyxiation method, followed by collection of aortic tissues for tissue staining to observe atherosclerotic plaques, lesion areas, and lipid deposition.
Oil Red O staining
Following fixation of slides of cells, aorta, or aortic root using 4% formaldehyde, they were stained with Oil Red O solution (01391, Sigma-Aldrich, St. Louis, MO, USA) for 30 minutes. Samples were then rinsed with 75% ethanol for 5 minutes and with phosphate-buffered saline (PBS), followed by immediate imaging. Oil Red O-positive cells were photographed using an optical microscope (Nikon, Tokyo, Japan).
Hematoxylin and eosin staining
Aortic tissues were attained at week 12 after modeling, fixed in 4% formaldehyde, and dehydrated in gradient alcohol. Following 2 xylene washes, aortic tissue sections were dewaxed, and hydrated, followed by hematoxylin and eosin (HE) staining according to the manuals of an HE staining kit (PT001; Bogoo, Shanghai, China). In detail, after 10 minutes of staining in hematoxylin at room temperature, the sections were hydrolyzed in 1% hydrochloric acid solution for 20 seconds, and then treated in 1% ammonia solution for 30 seconds. Subsequent to 3 minutes of eosin staining of the sections, the morphology of aortic atherosclerotic lesions was observed under the optical microscope.
Isolation and identification of VSMCs
Aortas were collected, and arterial fat and connective tissue were carefully isolated. The arteries were then cut into blocks of 1 mm in length. These sections were incubated for 20–25 minutes at 37 ℃ in a solution containing 1.5 mg/mL papain, 1.5 mg/mL threitol, and 1.5 mg/mL bovine serum albumin (BSA), followed by 6–10 minutes of treatment with 1.0 mg/mL collagenase, 1.5 mg/mL hyaluronidase, and 1.5 mg/mL BSA. After that, SMCs were separated by gentle grinding with a pipette. Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) comprising 20% fetal bovine serum (FBS) and 100 U/mL streptomycin/penicillin. Finally, immunofluorescence detection of α-smooth muscle actin (α-SMA) was conducted to identify VSMCs using primary rabbit antibody against α-SMA (1:500, Ab124964; Abcam, Cambridge, UK) and Alexa Fluor® 488-labeled secondary goat anti-mouse immunoglobulin G (IgG) (H+L) antibody (1:800, Ab150081; Abcam, UK).
Papain, threitol, BSA, collagenase, hyaluronidase, FBS, and streptomycin/penicillin were purchased from Solarbio (Beijing, China; G8430, D8220, A8010, C8140, H8030, S9030, and P1400, respectively).
In vitro cell model of ox-LDL-exposed VSMCs
The isolated VSMCs were stimulated with ox-LDL to develop in vitro cell model. The VSMCs received no treatment and served as controls, whilst ox-LDL-exposed VSMCs were untreated or treated with DMSO, genistein, BayK8644, nifedipine, genistein + BayK8644, short hairpin RNA (sh)-NC, sh-SRC, DMSO + oe-NC, genistein + oe-NC, genistein + oe-SRC, genistein + oe-LOX-1, oe-NC + sh-NC, oe-SRC + sh-NC, oe-SRC + sh-CACNA1C, sh-CACNA1C + oe-NC, or sh-CACNA1C + oe-LOX-1.
For the ox-LDL exposure, VSMCs were stimulated with 75 µg/mL of ox-LDL for 72 hours. The VSMCs were treated with 50 µmol/L genistein, 30 µmol/L BayK8644 (the L-Ca channel activator), and 50 µmol/L nifedipine (the L-Ca channel blocker) (HY-10588 and HY-B0284, respectively; MedChemExpress, Monmouth Junction, NJ, USA) 6 hours before ox-LDL exposure. Cells were grouped by liposome transfection with shRNA primers (sh-SRC, GCAAGATCACTAGACGGGAAT and sh-CACNA1C, CGCAGTCAAGTCTAATGTCTT) and pCMV6-AC-GFP (the overexpression vector, FH1215; Feng Hui Biotechnology Co., Ltd., Hunan, China). Transfection was performed using a lipofectamine 2000 kit (11668019, Thermo Fisher Scientific, Waltham, MA, USA). After 6 hours transfection, the medium was replaced with complete medium for 48 hours incubation. The cells were harvested to detect the transfection efficiency and utilized for subsequent experiments.
Western blot analysis
Total tissue or cellular protein was extracted using radio-immunoprecipitation assay (RIPA) lysis containing phenylmethylsulfonyl fluoride (P0013C; Beyotime, Shanghai, China), followed by 30 minutes incubation on ice and 10 minutes centrifugation at 4 ℃ and 8,000 g. Following the collection of the supernatant, the total protein concentration was measured with a bicinchoninic acid (BCA) kit. After 50 µg proteins were dissolved in 2× sodium dodecyl sulfate (SDS) loading buffer, each sample was subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electro-blotted to polyvinylidene fluoride (PVDF) membranes. Afterward, the membranes underwent 1 hour blocking with 5% skimmed milk powder at room temperature and probed with diluted primary antibodies (Abcam, UK) against SRC (1:1,000, ab133283, rabbit), phosphorylation (p)-SRC (1:5,000, ab185617, rabbit), CACNA1C (1:1,200, ab84814, mouse), LOX-1 (1: 1,000, ab214427, rabbit), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; ab9485, 1:2,500, loading control) overnight at 4 ℃. Next, the membranes were re-probed with horseradish peroxidase (HRP)-tagged secondary antibodies (Abcam, UK) against goat anti-rabbit IgG H&L (ab97051, 1:2,000) and goat anti-mouse IgG (H&L) (ab6789, 1:2,000) for 1 hour. The blots were visualized by enhanced chemiluminescence (ECL) kit (abs920, Absin) and photographed with a Bio-Rad image analysis system (Bio-Rad Laboratories, Hercules, CA, USA), followed by analysis with Quantity One v4.6.2 software (Bio-Rad, USA).
Patch-clamp experiments
Electrode solution (mM) comprised CsCl 110, Na2 phosphocreatine 20, egtazic acid (EGTA) 10, Na2ATP 5, MgCl2 5, NaGTP 0.2, and HEPES 10, with solution PH adjusted to 7.3 by CsOH. Extracellular bath solution (mM) was comprised of NaCl 135, BaCl2 10, TEA-Cl 10, HEPES 10, CsCl 5, and Glucose 10, with solution PH adjusted to 7.4 by CsOH. Experiments were carried out at room temperature. The glass microelectrode was made by 3–4 pulls of a microelectrode micropipette puller (Model P-97; Sutter Instrument, Novato, CA, USA) on the day of the experiment which was used with a resistance range of 4–7 MΩ. The cell membrane capacitance was in the range of 12.15±3.41 pF (n=96), with an access resistance >2 GΩ. The current at 10 mV was depolarized from −50 to +40 mV with a step of 500 ms, and current was recorded with a sampling frequency of 10 kHz at a holding potential of −70 mV. In this study, the whole-cell L-Ca current was recorded using a Axopatch 200B amplifier (Axon Instruments, Burlingame, CA, USA), Axon pCLAMP9.2 software, and digidata1322A interface (Axon Instruments, USA).
Cell Counting Kit-8 (CCK-8)
A CCK-8 kit (CK04; Dojindo Laboratories, Kumamoto, Japan) was applied for measurement of cell viability. Logarithmic growing cells were seeded at 1×104 per well in a 96-well plate for 24 hours. Cells were then grouped, and 10 µL CCK-8 reagents were added at 0, 24, 48, and 72 hours after grouping, and incubated at 37 ℃ for 3 hours. The absorbance values of each well at 450 nm were measured on a microplate reader, and the values were proportional to the number of cells proliferating in the culture medium. The growth curve was plotted.
Scratch test
The concentration of the cells was adjusted to 1×106 cells/mL with DMEM, and the cells were routinely cultured in 10% DMEM encompassing FBS until cell monolayers formed. The pipette tip was adopted to scratch along the bottom of the culture plate in a “1” shape, and the relative distance of the scratch area was recorded under a microscope. Then, the cells were washed with serum-free medium, followed by 24 hours of routine incubation. The cells were observed and photographed under an inverted microscope (Nikon, Japan) to assess the relative distance of cell migration to the wounded area. The actual cell migration distance was calculated as per the distance from the original cell injury area.
Immunofluorescence
The VSMCs were treated with PBS with the cell concentration adjusted to 1×106 cells/mL. Cells were supplemented with 20 µg/mL 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (Dil) cell membrane-labeled ox-LDL (Dil-ox-LDL) fluorescent dye for 4 hours incubation in a 37 ℃ incubator with 5% CO2 and saturation humidity. Following incubation, the intensity of Dil-ox-LDL fluorescence staining in cells was observed under an inverted fluorescence microscope (BX50; Olympus, Japan).
Enzymatic colorimetry
The cells were attained, and 1×106 cells were extracted with 200 µL cholesterol extract (trichloromethane: isopropanol: NP40 =7:11:0.1), followed by the determination of the cholesterol concentration. The remaining precipitates were lysed with 200 µL RIPA lysis buffer. Afterward, the protein concentration was estimated using the Lowry method, and the amount of cholesterol per mg of protein (µg/mg) was calculated.
Statistical analysis
All statistical analyses of the data were implemented using the software SPSS 21.0 (IBM Corp., Armonk, NY, USA). The measurement data were summarized as mean ± standard deviation and tested for normal distribution and homogeneity of variance. If the data conformed to normal distribution and homogeneity of variance, unpaired t-tests were utilized to compare data between the 2 groups. One-way analysis of variance (ANOVA) or repeated measures ANOVA was employed to compare data among multiple groups, followed by Tukey’s post-hoc test. A P value <0.05 indicated that the differences were statistically significant.
Results
Genistein alleviated AS in rats and inhibited ox-LDL-induced proliferation, migration, and foaming of VSMCs in vitro
To investigate the molecular mechanisms by which genistein affected AS, a rat model of AS was established. The results of Oil Red O staining and HE staining (Figure 1A-1C) showed that the atherosclerotic plaque lesion area and lipid deposition were significantly increased in rats after AS modeling, and that when AS rats were treated with genistein, the atherosclerotic plaque lesion area and lipid deposition were obviously decreased. This indicated that genistein could alleviate AS in rats.
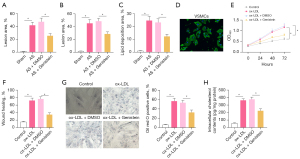
Rat VSMCs were isolated to develop an in vitro cell model. The VSMCs were identified by immunofluorescence detection of α-SMA in cells. The results (Figure 1D) displayed that VSMCs had green fluorescence, indicating their successful extraction. Next, VSMCs were stimulated with ox-LDL to develop an in vitro cell model. As reflected by the results of CCK-8 and scratch test (Figure 1E,1F, Figure S1A), ox-LDL strikingly enhanced cell viability and migration in VSMCs, whilst treatment with genistein markedly reduced the viability and migration of ox-LDL-exposed VSMCs. Uptake of large amounts of fat by VSMCs leads to cholesterol accumulation, which foams the cells, causing AS (20). Oil Red O staining (Figure 1G) demonstrated a dramatic increase of intracellular lipid particles in ox-LDL-exposed VSMCs and noticeable decline of intracellular lipid particles inox-LDL-exposed VSMCs following genistein treatment. Enzymatic colorimetry results (Figure 1H) documented that intracellular cholesterol content was substantially elevated in VSMCs by ox-LDL exposure, and that intracellular cholesterol content was conspicuously diminished in ox-LDL-exposed VSMCs after genistein treatment. These data suggested that genistein could inhibit ox-LDL-induced proliferation, migration, and foaming of VSMCs.
Genistein restrained ox-LDL-induced proliferation, migration, and foaming of VSMCs by decreasing L-Ca channel currents
Research has illustrated that genistein curtails L-Ca channels (21). To explore the influence of genistein on L-Ca channels, patch-clamp experiments were performed to detect L-Ca channel currents in VSMCs, which displayed that L-Ca channel currents were evidently augmented in VSMCs by ox-LDL exposure, which was nullified by genistein treatment (Figure 2A). Treatment of VSMCs with the L-Ca channel activator BayK8644 and blocker nifedipine revealed that cellular L-Ca channel currents were clearly increased after treatment with BayK8644 but decreased after treatment with nifedipine in ox-LDL-exposed VSMCs. Moreover, BayK8644 reversed the reduction of L-Ca channel currents by genistein in ox-LDL-exposed VSMCs (Figure 2B). Collectively, genistein suppressed ox-LDL-induced L-Ca channel currents in VSMC-derived foam cells.
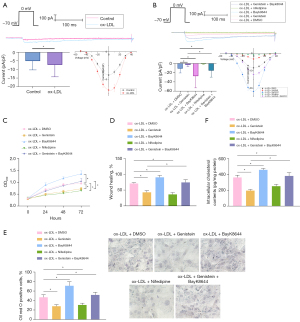
We further ascertained the effect of genistein on ox-LDL-exposed VSMC activities through inhibition of L-Ca channel currents. With respect to CCK-8 and scratch test results (Figure 2C,2D, Figure S1B), BayK8644 considerably enhanced but nifedipine prominently reduced ox-LDL-exposed VSMC viability and migration, and then BayK8644 annulled the decline of ox-LDL-exposed VSMC viability and migration induced genistein. As for the results of Oil Red O staining and enzymatic colorimetry (Figure 2E,2F), BayK8644 significantly increased intracellular lipid particles and cholesterol content in ox-LDL-exposed VSMCs, which was contrary after nifedipine treatment. BayK8644 abrogated the repressive impact of genistein on intracellular lipid particles and cholesterol content in ox-LDL-exposed VSMCs.
Conclusively, genistein could subdue ox-LDL-induced proliferation, migration, and foaming of VSMCs via the reduction of L-Ca channel currents.
Genistein suppressed ox-LDL-exposed VSMC proliferation, migration, and foaming by diminishing SRC activity
As tyrosine kinases, SRC family kinases have been indicated to assume a role in the atherosclerotic process (22). Since genistein is a tyrosine kinase inhibitor, we hypothesized that it could impact the activities of ox-LDL-induced VSMCs by repressing SRC activity. Western blot analysis results (Figure 3A,3B) exhibited that SRC phosphorylation level was significantly enhanced in the aortic tissues of rats by AS modeling, which was counteracted by genistein treatment. The SRC phosphorylation level was obviously elevated in VSMCs by ox-LDL exposure, which was negated after genistein treatment. This suggested that SRC was hyperphosphorylated in atherosclerotic tissues and that genistein could restrict SRC activity in AS.
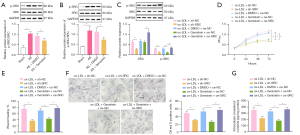
To dissect the influence of genistein on activities of ox-LDL-induced VSMCs by downregulating SRC, ox-LDL-induced VSMCs were treated with genistein, sh-SRC, or oe-SRC. Western blot analysis results (Figure 3C) documented appreciable reduction of SRC phosphorylation level in ox-LDL-induced VSMCs after silencing SRC or treatment with genistein. Furthermore, overexpression of SRC neutralized the inhibitory effect of genistein on SRC phosphorylation level. Besides, CCK-8 and scratch test (Figure 3D,3E, Figure S1C) depicted that silencing SRC or treatment with genistein observably lowered ox-LDL-induced VSMC proliferative and migratory capacity. Overexpression of SRC counterweighed the suppressive impacts of genistein on ox-LDL-induced VSMC proliferative and migratory capacity. The results of Oil Red O staining and enzymatic colorimetry (Figure 3F,3G) revealed diminished intracellular lipid particles and cholesterol content in ox-LDL-induced VSMCs after SRC silencing or genistein treatment. The decline of intracellular lipid particles and cholesterol content in ox-LDL-induced VSMCs triggered by genistein was undermined by overexpressing SRC.
In conclusion, genistein could downregulate SRC to restrict ox-LDL-induced VSMC proliferation, migration, and foaming.
Genistein repressed L-Ca channel currents by downregulate SRC in ox-LDL-induced VSMCs
Existing research has documented that SRC activates L-Ca channels (15). The results of patch-clamp experiments (Figure 4A) showed that cellular L-Ca channel currents were substantially decreased in ox-LDL-induced VSMCs after silencing SRC. Therefore, we speculate that genistein reduced L-Ca channel currents via SRC downregulation. After genistein treatment and SRC overexpression, patch-clamp experiments (Figure 4B) manifested conspicuous diminishment of cellular L-Ca channel currents in ox-LDL-induced VSMCs following genistein treatment, which was nullified by further overexpressing SRC.
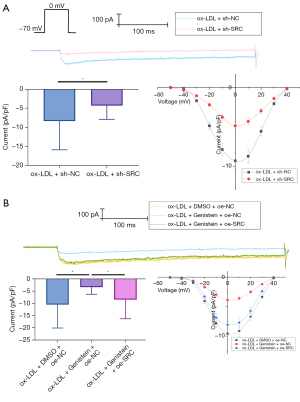
In summary, genistein could curtail L-Ca channel currents by reducing SRC activity.
SRC facilitated ox-LDL-induced proliferation, migration, and foaming of VSMCs by acting on the L-Ca channel subunit CACNA1C
As a subunit of the L-Ca channel, CACNA1C has been reported to be involved in the process of AS (17,23). Hence, a hypothesis was proposed that SRC might act on CACNA1C. Based on western blot analysis results (Figure 5A,5B), AS modeling conspicuously augmented CACNA1C protein expression in aortic tissues of rats. In VSMCs, ox-LDL induction evidently increased CACNA1C protein expression, which was neutralized by additional SRC silencing. The aforesaid results indicated that CACNA1C was highly expressed in atherosclerotic tissues of rats, and that silencing SRC could lower CACNA1C protein expression in ox-LDL-induced VSMCs.
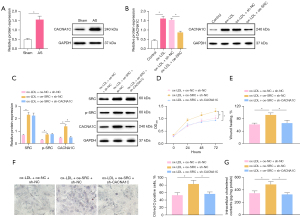
To evaluate whether SRC affected the activities of ox-LDL-induced VSMCs via CACNA1C, SRC was overexpressed and CACNA1C was silenced in ox-LDL-induced VSMCs. As indicated by western blot analysis results (Figure 5C), SRC and CACNA1C protein expression were signally elevated in ox-LDL-induced VSMCs following overexpression of SRC, and CACNA1C protein expression considerably decreased in ox-LDL-induced VSMCs after silencing CACNA1C in the presence of SRC overexpression. For the results of CCK-8 and scratch test (Figure 5D,5E, Figure S1D), the noticeable increase of cell proliferation and migration was observed in ox-LDL-induced VSMCs subsequent to SRC overexpression, whilst these trends were abolished by further silencing CACNA1C. Oil Red O staining and enzymatic colorimetry results (Figure 5F,5G) demonstrated that SRC overexpression caused the prominent elevation of intracellular lipid particles and cholesterol content, which was counteracted after further silencing CACNA1C.
Taken together, SRC could accelerate the proliferation, migration, and foaming of ox-LDL-induced VSMCs by acting on the L-Ca channel subunit CACNA1C.
Silencing CACNA1C restricted ox-LDL-induced proliferation, migration, and foaming of VSMCs through downregulation of LOX-1
Existing literature has demonstrated that CACNA1C facilitates cellular uptake of ox-LDL by upregulating LOX-1 (18). Western blot analysis (Figure 6A,6B) exhibited that LOX-1 protein expression was evidently elevated in aortic tissues of rats after AS modeling and in VSMCs following ox-LDL induction. In ox-LDL-exposed VSMCs, LOX-1 protein expression considerably diminished after silencing CACNA1C, which was annulled by further overexpression of LOX-1. The data revealed that LOX-1 expression was high in atherosclerotic tissues of rats and that silencing CACNA1C resulted in downregulation of LOX-1.
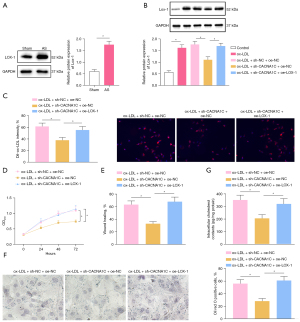
Immunofluorescence (Figure 6C) manifested that CACNA1C silencing prominently lowered cellular uptake of Dil-ox-LDL by VSMCs, which was negated after additional LOX-1 overexpression. The results of CCK-8 and scratch test (Figure 6D,6E, Figure S1E) displayed the marked reduction of ox-LDL-exposed VSMC proliferation and migration after silencing of CACNA1C, which was counteracted following further overexpression of LOX-1. The results of Oil Red O staining and enzymatic colorimetry (Figure 6F,6G) depicted that CACNA1C silencing appreciably decreased intracellular lipid particles and cholesterol content in ox-LDL-exposed VSMCs and that additional LOX-1 overexpression abrogated these trends.
In summary, CACNA1C silencing reduced the uptake of ox-LDL by VSMCs by downregulating LOX-1, thereby repressing ox-LDL-induced proliferation, migration, and foaming of VSMCs.
Overexpression of LOX-1 counterweighed the ameliorative impacts of genistein on AS in rats and ox-LDL on VSMCs
To further elucidate the mechanism by which genistein alleviated AS in vivo, AS rats were treated with genistein and oe-LOX-1. Western blot analysis (Figure 7A) manifested that genistein treatment of AS rats strikingly lowered SRC phosphorylation level and CACNA1C and LOX-1 protein expression in aortic tissues. Meanwhile, LOX-1 overexpression reversed the decrease of LOX-1 protein expression triggered by genistein without impacts on SRC phosphorylation level and CACNA1C protein expression. As illustrated by the results Oil Red O staining and HE staining (Figure 7B-7D), observably decreased atherosclerotic plaque lesion areas and lipid deposition were found in AS rats after genistein treatment, which was neutralized by additional overexpression of LOX-1.
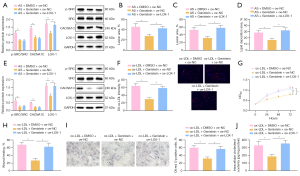
The ox-LDL-exposed VSMCs were treated with genistein and oe-LOX-1. Western blot analysis (Figure 7E) showed that genistein treatment dramatically lowered SRC phosphorylation level and CACNA1C and LOX-1 protein expression in ox-LDL-exposed VSMCs. In the presence of genistein, overexpression of LOX-1 augmented LOX-1 protein expression but did not affect SRC phosphorylation level and CACNA1C protein expression in ox-LDL-exposed VSMCs. For immunofluorescence results (Figure 7F), cellular uptake of Dil-ox-LDL by VSMCs was observably reduced after genistein treatment, which was noticeably nullified through further overexpression of LOX-1. The CCK-8 and scratch test results demonstrated that ox-LDL-exposed VSMC proliferation and migration were markedly diminished following genistein treatment, which was abrogated after additional LOX-1 overexpression (Figure 7G,7H, Figure S1F). Oil Red O staining and enzymatic colorimetry showed the diminishment in intracellular lipid particles and cholesterol content inox-LDL-exposed VSMC after genistein treatment and that these results were negated by further overexpressing LOX-1 (Figure 7I,7J).
Together, LOX-1 upregulation abolished the alleviatory impacts of genistein on AS in rats and ox-LDL on VSMCs.
Discussion
Genistein supplementation is an attractive alternative for cardiovascular protection based on previous epidemiological and animal model studies (24). The study of Thangavel et al. has highlighted inhibitory effect of genistein on incidence of cardiovascular disease, osteoporosis, and the postmenopausal symptoms (25). Some ideas have been presented by Si et al. demonstrating the possible mechanisms of the genistein action on vascular protective effects, where genistein may attenuate pro-inflammatory factor-induced vascular endothelial barrier dysfunction, inactivate the cAMP/PKA cascade, activate peroxisome proliferator-activated receptors, or reduce production of reactive oxygen species-producing enzymes (26). However, few studies exist to provide a clear mechanistic understanding of the molecular mechanistic basis of genistein in AS. Based on this, this research set out to ascertain the mechanism of genistein in AS, and the obtained data revealed that genistein might reduce the uptake of ox-LDL by VSMCs through inhibiting SRC action on the L-Ca channel subunit CACNA1C to downregulate LOX-1, thereby suppressing ox-LDL-induced proliferation, migration, and foaming of VSMCs and preventing the formation of AS (Figure 8).
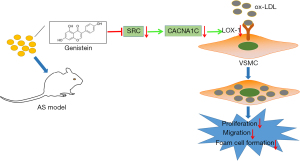
The initial finding in our research was that genistein alleviated AS in rats. Similarly, mounting evidence has illustrated the atheroprotective impacts of genistein. For instance, it has been displayed that genistein can repress tumor necrosis factor-α (TNF-α)-triggered adhesion of monocytes to human umbilical vein endothelial cells, a crucial event in AS pathogenesis (27). In addition, a prior work had indicated that a genistein derivative, 7-Difluoromethyl-5,4'-dimethoxygenistein, can lower lipid plasma levels to curtail AS development (28). On the grounds of ‘inflammation’ hypotheses of AS formation, anti-inflammatory agents hold remarkable potential for AS management (29). Of interest, genistein has been suggested to present anti-inflammatory properties in VSMCs of atherosclerosis lesions, which were realized by regulating the ER-p38/ERK1/2-PPARγ-NF-κB-CRP/MMP-9 axis (30). Moreover, another study revealed that genistein causes significant declines in the formation of atherosclerotic plaques in LDL receptor-/- rats by downregulating inflammatory NF-kB and VCAM-1 (31).It should be noted that VSMCs are a primary cell type existing at all stages of atherosclerotic plaques (32).
In the subsequent assays, we observed that genistein diminished VSMC proliferation, migration, and foaming caused by ox-LDL. Consistent with our results, the research performed by Bai et al. elucidated that genistein restricted the proliferation of ox-LDL-induced VSMCs (11). Besides, a prior work unraveled that genistein resulted in the decrease in VSMC proliferation and migration during neointima formation (33). Genistein could suppress VSMC-derived foam cell formation through the downregulation of LOX-1 by blocking tyrosine kinase pathway (34). Furthermore, our work exhibited that inhibition of L-Ca channels participated in the suppressive impacts of genistein on ox-LDL-induced VSMC proliferation, migration, and foaming. Concordant with our findings, it was noted in a previous study that genistein restrained L-Ca channels in gastrointestinal smooth muscle (35). Of note, the L-Ca channel blocker, diltiazem, has been documented to reduce VSMC migration during AS (36). Also, it was previously reported that another L-Ca channel blocker, nifedipine, exerted anti-proliferative effects on rat aortic VSMCs (37), which is in line with our results. Hence, genistein suppressed L-Ca channels to diminish ox-LDL-induced VSMC proliferation, migration, and foaming.
Another finding of our study was that genistein restricted ox-LDL-induced VSMC proliferation, migration, and foaming by inhibiting SRC activity. This finding is partially supported by a report by Lee et al. that genistein obviously decreased SRC phosphorylation in prostate cancer cells (38). A recent report unveiled that repressing SRC activation attenuated ox-LDL-induced lipid accumulation and foam cell formation in VSMCs (39). Concordantly, Lang et al. demonstrated that the downregulation of SRC correlated to the reduced proliferation and migration of hydrogen peroxide-exposed VSMCs (40). Notably, another study manifested that c-SRC could induce the migration of VSMCs through activating L-Ca channels (8), which is in parallel with our results that genistein suppressed L-Ca channels by reducing SRC activity.
Besides, we observed that SRC accelerated ox-LDL-triggered proliferation, migration, and foaming of VSMCs via LOX-1 downregulation by acting on CACNA1C. As an L-Ca channel subunit (23), CACNA1C has been implicated in AS formation (17). Moreover, the same relationship between CACNA1C and LOX-1 was illustrated in a previous study (18). More importantly, the anti-atherosclerotic activity of LOX-1 has been found by the research of Li et al. (41). Concurrently, a recent work exhibited that LOX-1 overexpression was associated with VSMC proliferation caused by ox-LDL (42). As previously reported, LOX-1 downregulation culminated in restricted ox-LDL-exposed foam cell formation in rat aortic VSMCs, thus exerting an ameliorating effect in AS formation (43,44). In alignment with our findings, LOX-1 upregulation was involved in ox-LDL-triggered VSMC proliferation, migration, and foam cell formation (45).
Taken together, our study adds to the growing evidence for the atheroprotective effects of genistein. Specifically, genistein protected VSMCs against ox-LDL-exposed proliferation, migration, and foam cell formation through LOX-1 downregulation by blocking the action of SRC on the L-Ca channel subunit CACNA1C, which alleviated AS. Investigation of genistein and the SRC/CACNA1C/LOX-1 axis in VSMCs and AS yields a better understanding of their in vivo mechanisms and may have potentially critical therapeutic implications in the treatment of AS.
Acknowledgments
Funding: This study was funded by the National Natural Science Fund (No. 81471205).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2113/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2113/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2113/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were performed under a project license (No. 2021086) granted by Animal Ethical Care Committee of the First Affiliated Hospital of Harbin Medical University, in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kobiyama K, Ley K. Atherosclerosis. Circ Res 2018;123:1118-20. [Crossref] [PubMed]
- Libby P. The changing landscape of atherosclerosis. Nature 2021;592:524-33. [Crossref] [PubMed]
- Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med 2013;11:117. [Crossref] [PubMed]
- Zhu Y, Xian X, Wang Z, et al. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018;8:80. [Crossref] [PubMed]
- Chistiakov DA, Melnichenko AA, Myasoedova VA, et al. Mechanisms of foam cell formation in atherosclerosis. J Mol Med (Berl) 2017;95:1153-65. [Crossref] [PubMed]
- Wang Z, Guo X, Zhang Q, et al. Elimination of Ox-LDL through the liver inhibits advanced atherosclerotic plaque progression. Int J Med Sci 2021;18:3652-64. [Crossref] [PubMed]
- Pryma CS, Ortega C, Dubland JA, et al. Pathways of smooth muscle foam cell formation in atherosclerosis. Curr Opin Lipidol 2019;30:117-24. [Crossref] [PubMed]
- Guo X, Kashihara T, Nakada T, et al. PDGF-induced migration of synthetic vascular smooth muscle cells through c-Src-activated L-type Ca2+ channels with full-length CaV1.2 C-terminus. Pflugers Arch 2018;470:909-21. [Crossref] [PubMed]
- Mukund V, Mukund D, Sharma V, et al. Genistein: Its role in metabolic diseases and cancer. Crit Rev Oncol Hematol 2017;119:13-22. [Crossref] [PubMed]
- Wei J, Yao Z, Li H, et al. Genistein alleviates atherosclerosis in apolipoprotein E-deficient mice by interrupting the OX40/OX40L pathway. Int J Clin Exp Pathol 2019;12:1658-65. [PubMed]
- Bai B, Lu N, Zhang W, et al. Inhibitory Effects of Genistein on Vascular Smooth Muscle Cell Proliferation Induced by Ox-LDL: Role of BKCa Channels. Anal Cell Pathol (Amst) 2020;2020:8895449. [Crossref] [PubMed]
- Ono M, Takeshima M, Nishi A, et al. Genistein Suppresses v-Src-Driven Proliferative Activity by Arresting the Cell-Cycle at G2/M through Increasing p21 Level in Src-Activated Human Gallbladder Carcinoma cells. Nutr Cancer 2021;73:1471-9. [Crossref] [PubMed]
- Bagnato G, Leopizzi M, Urciuoli E, et al. Nuclear Functions of the Tyrosine Kinase Src. Int J Mol Sci 2020;21:2675. [Crossref] [PubMed]
- Wang JG, Aikawa M. Toll-like receptors and Src-family kinases in atherosclerosis -- focus on macrophages. Circ J 2015;79:2332-4. [Crossref] [PubMed]
- Kashihara T, Nakada T, Kojima K, et al. Angiotensin II activates CaV 1.2 Ca2+ channels through β-arrestin2 and casein kinase 2 in mouse immature cardiomyocytes. J Physiol 2017;595:4207-25. [Crossref] [PubMed]
- Kabir ZD, Martínez-Rivera A, Rajadhyaksha AM. From Gene to Behavior: L-Type Calcium Channel Mechanisms Underlying Neuropsychiatric Symptoms. Neurotherapeutics 2017;14:588-613. [Crossref] [PubMed]
- Peng C, Ding Y, Yi X, et al. Association study of CACNA1C polymorphisms with large artery atherosclerotic stroke in Chinese Han population. Neurol Res 2018;40:677-82. [Crossref] [PubMed]
- Komoda H, Shiraki A, Oyama JI, et al. Azelnidipine Inhibits the Differentiation and Activation of THP-1 Macrophages through the L-Type Calcium Channel. J Atheroscler Thromb 2018;25:690-7. [Crossref] [PubMed]
- Cheng XL, Ding F, Wang DP, et al. Hexarelin attenuates atherosclerosis via inhibiting LOX-1-NF-κB signaling pathway-mediated macrophage ox-LDL uptake in ApoE-/- mice. Peptides 2019;121:170122. [Crossref] [PubMed]
- Yu XH, Chen JJ, Deng WY, et al. Biochanin A Mitigates Atherosclerosis by Inhibiting Lipid Accumulation and Inflammatory Response. Oxid Med Cell Longev 2020;2020:8965047. [Crossref] [PubMed]
- Belevych AE, Warrier S, Harvey RD. Genistein inhibits cardiac L-type Ca(2+) channel activity by a tyrosine kinase-independent mechanism. Mol Pharmacol 2002;62:554-65. [Crossref] [PubMed]
- Yin C, Vrieze AM, Rosoga M, et al. Efferocytic Defects in Early Atherosclerosis Are Driven by GATA2 Overexpression in Macrophages. Front Immunol 2020;11:594136. [Crossref] [PubMed]
- Hedley PL, Jørgensen P, Schlamowitz S, et al. The genetic basis of Brugada syndrome: a mutation update. Hum Mutat 2009;30:1256-66. [Crossref] [PubMed]
- Dixon RA, Ferreira D. Genistein. Phytochemistry 2002;60:205-11. [Crossref] [PubMed]
- Thangavel P, Puga-Olguín A, Rodríguez-Landa JF, et al. Genistein as Potential Therapeutic Candidate for Menopausal Symptoms and Other Related Diseases. Molecules 2019;24:3892. [Crossref] [PubMed]
- Si H, Liu D. Phytochemical genistein in the regulation of vascular function: new insights. Curr Med Chem 2007;14:2581-9. [Crossref] [PubMed]
- Jia Z, Babu PV, Si H, et al. Genistein inhibits TNF-α-induced endothelial inflammation through the protein kinase pathway A and improves vascular inflammation in C57BL/6 mice. Int J Cardiol 2013;168:2637-45. [Crossref] [PubMed]
- Zhang Y, Li L, You J, et al. Effect of 7-difluoromethyl-5, 4'-dimethoxygenistein on aorta atherosclerosis in hyperlipidemia ApoE(-/-) mice induced by a cholesterol-rich diet. Drug Des Devel Ther 2013;7:233-42. [Crossref] [PubMed]
- Xu S, Pelisek J, Jin ZG. Atherosclerosis Is an Epigenetic Disease. Trends Endocrinol Metab 2018;29:739-42. [Crossref] [PubMed]
- Xu L, Liu JT, Li K, et al. Genistein inhibits Ang II-induced CRP and MMP-9 generations via the ER-p38/ERK1/2-PPARγ-NF-κB signaling pathway in rat vascular smooth muscle cells. Life Sci 2019;216:140-6. [Crossref] [PubMed]
- Wang J, Zhang R, Xu Y, et al. Genistein inhibits the development of atherosclerosis via inhibiting NF-kappaB and VCAM-1 expression in LDLR knockout mice. Can J Physiol Pharmacol 2008;86:777-84. [Crossref] [PubMed]
- Basatemur GL, Jørgensen HF, Clarke MCH, et al. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol 2019;16:727-44. [Crossref] [PubMed]
- Tsai YC, Leu SY, Peng YJ, et al. Genistein suppresses leptin-induced proliferation and migration of vascular smooth muscle cells and neointima formation. J Cell Mol Med 2017;21:422-31. [Crossref] [PubMed]
- Lin J, Xu Y, Zhao T, et al. Genistein suppresses smooth muscle cell-derived foam cell formation through tyrosine kinase pathway. Biochem Biophys Res Commun 2015;463:1297-304. [Crossref] [PubMed]
- Zhang LX, Li HF, Wang LD, et al. Resveratrol and genistein inhibition of rat isolated gastrointestinal contractions and related mechanisms. World J Gastroenterol 2014;20:15335-42. [Crossref] [PubMed]
- Liu W, Hashimoto T, Yamashita T, et al. Coagulation factor XI induces Ca2+ response and accelerates cell migration in vascular smooth muscle cells via proteinase-activated receptor 1. Am J Physiol Cell Physiol 2019;316:C377-92. [Crossref] [PubMed]
- Sung JY, Choi HC. Nifedipine inhibits vascular smooth muscle cell proliferation and reactive oxygen species production through AMP-activated protein kinase signaling pathway. Vascul Pharmacol 2012;56:1-8. [Crossref] [PubMed]
- Lee J, Ju J, Park S, et al. Inhibition of IGF-1 signaling by genistein: modulation of E-cadherin expression and downregulation of β-catenin signaling in hormone refractory PC-3 prostate cancer cells. Nutr Cancer 2012;64:153-62. [Crossref] [PubMed]
- Chen Z, Xue Q, Cao L, et al. Toll-Like Receptor 4 Mediated Oxidized Low-Density Lipoprotein-Induced Foam Cell Formation in Vascular Smooth Muscle Cells via Src and Sirt1/3 Pathway. Mediators Inflamm 2021;2021:6639252. [Crossref] [PubMed]
- Lang Y, Chen D, Li D, et al. Luteolin inhibited hydrogen peroxide-induced vascular smooth muscle cells proliferation and migration by suppressing the Src and Akt signalling pathways. J Pharm Pharmacol 2012;64:597-603. [Crossref] [PubMed]
- Li Q, Zhao W, Zeng X, et al. Ursolic Acid Attenuates Atherosclerosis in ApoE-/- Mice: Role of LOX-1 Mediated by ROS/NF-κB Pathway. Molecules 2018;23:1101. [Crossref]
- Zhang Z, Zhang D, Du B, et al. Hyperoside inhibits the effects induced by oxidized low-density lipoprotein in vascular smooth muscle cells via oxLDL-LOX-1-ERK pathway. Mol Cell Biochem 2017;433:169-76. [Crossref] [PubMed]
- Cheng CI, Tai MH, Chang HR, et al. Oxidized low-density lipoprotein induced hepatoma-derived growth factor upregulation mediates foam cell formation of cultured rat aortic vascular smooth muscle cells. Eur J Cell Biol 2021;100:151169. [Crossref] [PubMed]
- Zhang D, Gao JL, Zhao CY, et al. Cyclin G2 promotes the formation of smooth muscle cells derived foam cells in atherosclerosis via PP2A/NF-κB/LOX-1 pathway. Ann Transl Med 2021;9:446. [Crossref] [PubMed]
- Li W, Zhi W, Zhao J, et al. Cinnamaldehyde protects VSMCs against ox-LDL-induced proliferation and migration through S arrest and inhibition of p38, JNK/MAPKs and NF-κB. Vascul Pharmacol 2018;108:57-66. [Crossref] [PubMed]
(English Language Editor: J. Jones)


