
Aldosterone/direct renin concentration ratio as a screening test for primary aldosteronism: a systematic review and meta-analysis
Introduction
Primary aldosteronism (PA) is a group of clinical syndromes caused by excessive secretion of aldosterone by the adrenal cortex. The main clinical manifestations are episodic weakness, increased blood pressure, and hypokalemia. Aldosterone-induced water and sodium retention is the direct cause of secondary hypertension. According to published reports, more than 10% of hypertensive patients are caused by PA (1,2), the prevalence of PA is very high, at least 4% among newly diagnosed hypertensive patients in China (3). Excessive aldosterone can target and damage vital organs in the body, such as the heart, brain and kidneys, especially by increasing the duration of cardiovascular disease and impairing kidney function (2,4,5). Hyperaldosteronism may also lead to impaired glucose tolerance and osteoporosis (6,7). As the global population ages, the World Health Organization (WHO) predicts that, by 2050, the global population of people aged 60 and over will increase substantially, possibly twice as much (8,9). Aging, which means more hypertensive patients, draws further attention to PA screening. Early screening for PA in young and middle-aged population is of great significance for delaying complications and reducing medical burden.
At the same time, the treatment of PA is different from the treatment of essential hypertension, so it is extremely important to screen for hypertension caused by PA. Currently, the plasma aldosterone-to-renin ratio (ARR) is a commonly used PA screening index, calculated based on plasma aldosterone concentration and renin activity measured by radioimmunoassay (5,10-12). But, the radioimmunoassay has the disadvantages of indirect estimation, possible influence by plasma angiotensinogen concentration, generation of radioactive toxic substances, and complicated operation. ARR is also altered by age-induced changes in plasma renin and aldosterone levels, which affects the diagnostic accuracy of screening (9,13). In recent years, although the clinical application of aldosterone to direct renin concentration ratio (ADRR) is still in progress, because ADRR has the advantages of good repeatability, simple operation, and stable results, the accuracy of screening for PA is higher. Scholars have proposed using ADRR to replace radioimmunoassay and ARR as a screening test for PA, which has attracted much attention (14-16).
However, the diagnostic efficiency of ADRR varies among studies and there is no general consensus (9,12,15,17,18). The detection method of direct renin concentration is constantly improving. Meta-analysis can summarize existing study and discover ADRR and PA correlations. Therefore, we conducted a meta-analysis of relevant clinical studies in recent years to evaluate the accuracy of ADRR in PA screening and provide more references for clinical application. We present the following article in accordance with the PRISMA reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2272/rc).
Methods
Search strategy
The English electronic databases of PubMed, Embase, and Cochrane Library, and the Chinese electronic databases China National Knowledge Infrastructure (CNKI), Wanfang, and Weipu libraries were screened. “Primary aldosteronism”, “primary hyperaldosteronism”, “aldosterone”, “renin concentration”, “hypertension”, and “screening test” were used as the keywords to search the database as mentioned above. The search was limited to English and Chinese publications involving human participants. Articles included were drawn from the aforementioned electronic databases from January 2014 to August 2021, and the related bibliography of published articles was screened for relevant additional articles.
Inclusion criteria
When the retrieved article met the following conditions, it was included in this study: (I) according to the PICOS principles, the research objects are healthy people, patients with hypertension, and PA patients; (II) intervention measures are the guideline-recommended 4 confirmatory tests include four, the first being an oral sodium loading test, the second a saline infusion test, the third a fludrocortisone suppression test, and a final captopril challenge test, the study was conducted with at least one of these 4 tests, or a histopathological diagnosis of PA; (III) a study on the diagnostic accuracy comparison of ADRR in PA screening can be constructed with a specific cut-off value research; (IV) full-text publication available; (V) article outcome data could be completely extracted, and the research type is random control research.
Paper screening and data extraction
Abstracts were independently evaluated by 2 review authors according to pre-specified inclusion criteria. When there is disagreement about the article, a third review author was invited to arbitrate. Data extraction was also performed independently for all selected research articles by these 2 review authors. The extracted data included the first author, country of origin, year of publication, sample size, gender, original cut-off value of positive results, antihypertensive drug status, the patient’s physiology at the time of blood collection, the concentration of potassium in the blood, and true positives, true negatives, false positives, and false negatives analyzed. If there is a disagreement during the data extraction process, resolve the disagreement through discussion until a consensus is reached. If the required data was not clear or not provided in the full text, we contacted the author to obtain more data or information; if the relevant data was not available, the article was eliminated.
Quality assessment
The quality of the articles included in this meta-analysis was assessed according to the Diagnostic Accuracy Study 2 (QUADAS-2) criteria, and the research methods in each included article were assessed for quality, as recommended by the Cochrane Collaboration. Simply put, the QUADAS-2 standard consists of 4 key areas: patient selection, index testing, reference standards, and the flow and time of samples/patients in the study. The evaluation results of the above indicators are divided into three levels, namely, good “low risk”, poor “high risk” or “unclear”.
Statistical analysis
Spearman’s correlation coefficient was used to evaluate the threshold effect. In addition, in order to assess the heterogeneity between studies, I2 was used for impact analysis. An I2 of 0% indicates unobserved heterogeneity. An I2 over 50% may represent significant heterogeneity. In this study, the random effects model was used when I2 was greater than 30%, otherwise the fixed effects model was used. The aggregate sensitivity and specificity estimated cut-off points with a 95% confidence interval (CI) were calculated for PA screening. The forest plot was used to show the sensitivity and specificity of the indicators in this study. For the accuracy of each result, a summary receiver operating characteristic (SROC) curve and respective area under the curve (AUC) were constructed for analysis. When the critical P value is set to 0.05, when it is greater than 0.05, it is considered that the difference is not statistically significant, otherwise it is significant.
Results
Search results and study characteristics
After searching the electronic database, 794 articles were obtained. After understanding the research content of the articles through the title and abstract, 157 articles were considered eligible for full-text evaluation. The reasons for the exclusion of the other articles were as follows: (I) the article could not provide a specific cut-off value (n=47); (II) the article did not describe which confirmatory test was used to diagnose PA (n=38); (III) the study subject took captopril (n=62), as shown in Figure 1.
Among the 10 included studies (9,12,15,17-23) there were 2,806 participants, with 10 articles published between 2014 and 2021. The sample size included in a single study ranged from 75 to 542 cases, 2 of which was in Chinese and 8 in English. PA patients were included in the study arm in each study, and one study included the PA subtype, aldosterone adenoma (APA), as the study arm, and normotensive or essential hypertension (EH) patients as the control arm. The relevant features are shown in Table 1.
Table 1
| Author | Country | Year | Journal | Sample size | Cut-off (original test) | Cut-off (ng/dL)/(mU/L) |
|---|---|---|---|---|---|---|
| Ma et al. (9) | China | 2018 | Int J Endocrinol | 485 | 3.7 (ng/dL)/(μIU/mL) | 3.7 |
| Gan et al. (12) | China | 2019 | J Hum Hypertens | 442 | 28.3 (pg/mL)/(pg/mL) | 6.2 |
| Lonati et al. (15) | Italy | 2014 | J Hypertens | 88 | 3.7 (ng/dL/mU/L) | 3.7 |
| Li et al. (17) | China | 2019 | Int J Hypertens | 450 | 2.93 (ng/dL)/(mU/L) | 2.9 |
| Qin et al. (18) | China | 2020 | Chin J Hypertens | 183 | 4.4 (ng/dL)/(mU/L) | 4.4 |
| Teruyama et al. (19) | Japan | 2022 | J Hum Hypertens | 75 | 1.31 (ng/dL)/(pg/mL) | 2.9 |
| Morimoto et al. (20) | Japan | 2017 | Hypertension | 125 | 6.0 (ng/dL)/(pg/mL) | 13.2 |
| Jansen et al. (21) | Groningen | 2014 | J Hypertens | 178 | 91 (pmol/L)/(mU/L) | 3.3 |
| Ma et al. (22) | China | 2020 | Natl Med J China | 542 | 7.8 (ng/L)/(mU/L) | 7.8 |
| Lin et al. (23) | China | 2020 | Int J Hypertens | 238 | 28 (pg/mL)/(pg/mL) | 6.2 |
Conversion factors: aldosterone concentration, 1 ng/dL =27.7 pmol/L; renin concentration, 1 mU/L =2.2 pg/mL.
Risk of bias and applicability judgments
Among the risk of bias, all articles had a low risk of bias when selecting patients. As for the index test, 3 studies had high risk because they did not pre-specify the cut-off value of ADRR, and the remaining 7 studies were low risk. None of the studies on reference standards showed high risks. All studies on flow and time were also low risk. In the applicability assessment, no high risk of bias was found in the results of related indicators. The result of the risk assessment is shown in Figure 2.
Overall analyses
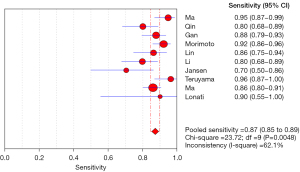
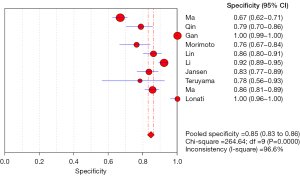
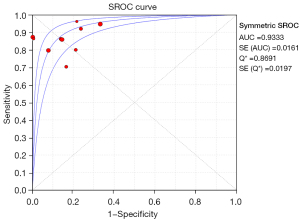
Meta regression analysis showed that the pooled sensitivity of aldosterone/direct renin concentration ratio for screening PA was 0.87 (95% CI: 0.85–0.89) (Figure 3), and the pooled specificity was 0.85 (95% CI: 0.83–0.86) (Figure 4). In addition, the sensitivity and specificity I2 were 62.1% and 96.6%, respectively. The SROC showed high accuracy [summary (S)AUC =0.9333] (Figure 5), the closer the AUC of SROC was to 1.0, the more authentic the detection method was.
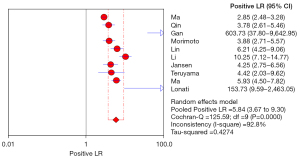
The positive likelihood ratio (PLR) is the multiple that a correct diagnosis of a disease is a wrong diagnosis of a disease in a diagnostic experiment. Therefore, the greater the ratio of the PLR, the higher the accuracy of the diagnosis. In this study, the pooled PLR of ADRR in the diagnosis of PA was 5.84 (3.67–9.30), indicating that ADRR is more reliable as a PA screening test, as shown in Figure 6.
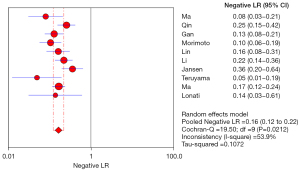
Negative Likelihood ratio (NLR) is a multiple of the probability of wrongly diagnosing a negative disease, and it can also be understood as a multiple of the negative probability of correctly diagnosing a disease, and is an important reference for analysis. Therefore, the smaller the NLR, the higher the accuracy of the diagnostic test. The pooled NLR in this study was 0.16 (0.12–0.22), which indicates that the ADRR screening experiment was more accurate for PA screening, as shown in Figure 7.
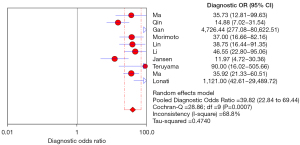
The diagnostic odds ratio (DOR) is also an important analytical reference, representing how closely the results of a diagnostic test are related to the disease being diagnosed. The larger the value, the greater the ability of the corresponding disease to be diagnosed. In this study, the results of the analysis showed that the combined DOR was 39.82 (22.84–69.44). It can be considered that the authenticity of the ADRR screening and diagnosis of PA is strong, as shown in Figure 8.
Risk of bias
Among the available articles’ risk of bias, all articles had a lower risk of bias when selecting patients. A total of 3 studies had a unclear risk of index test because they did not prespecify the critical value of ADRR (12,19,20), and the index test of the remaining 7 studies was low risk (9,15,17,18,21-23). All research reference standards, flow, and time were low risk. None of the applicability bias indicators of all studies found a high risk of bias, as shown in Figure 9.
Discussion
Endocrine hypertension is commonly caused by PA. It is mainly due to adrenal cortex lesions that lead to a large amount of aldosterone secretion. Too much aldosterone can lead to water and sodium retention in the body, increased intravascular blood volume, and so on, which can lead to high blood pressure (4,14), with or without clinical manifestations such as hypokalemia. It has been shown by recent study that increased aldosterone can also lead to impaired glucose tolerance and changes in homeostasis of plasma Mg2+, Ca2+ and Cl− (24). International researchers such as Vaidya have reported that due to the autogenous excessive secretion of aldosterone caused by PA, its main role is to preserve sodium and excrete potassium, activate mineralocorticoid receptor (MR), and activate MR in the distal nephrons of the kidney (25). Induces a series of reactions, and causes the reabsorption of sodium ions by acting on the epithelial sodium channels, causing increased blood volume, hypertension, and inhibition of renin.
Due to its high risk of cerebrocardiovascular and renal diseases, with the use of ARR for its screening, the detection rate of PA has increased significantly compared with before, which has attracted widespread attention (25-28). Therefore, it is extremely important to screen hypertensive patients with primary aldosteronism at an early stage, which can reduce the risk of cardiovascular, kidney, or other important organ damage (15,27). However, as a sensitive indicator for screening PA, the guidelines do not have uniform ARR diagnostic thresholds and screening strategies. Although standing ADRR is the most important indicator for PA screening, it should be combined with the measurement results of renin and aldosterone (29). Plasma aldosterone levels may not be significantly elevated in patients with IHA, and medications may affect renin levels. Focusing only on ADRR may lead to misjudgment of results.
ADRR is used to screen for PA, and reports have historically varied in terms of accuracy. Therefore, we conducted this meta-analysis to assess its accuracy and provide reference. The meta-analysis showed that for PA screening, the sensitivity and specificity of ADRR were 0.87 (95% CI: 0.85–0.89) and 0.85 (95% CI: 0.83–0.86), respectively. At the same time, the analysis results confirmed that ADRR screening PA has a higher PLR and a lower NLR. The diagnostic accuracy is at least comparable to previous PRA-based ARR study (14). In addition, the DOR also showed that ADRR is an effective test. According to the QUADAS-2 standard, the research quality of this analysis was good, indicating that the summary results are of great reference value.
This meta-analysis had several limitations. In research, sensitivity and specificity are easy to understand and widely accepted by clinicians, so sensitivity and specificity were used as the main outcome indicators, plus PLR and NLR as outcome indicators. However, due to the lack of a gold standard for PA diagnosis, the included research articles did not have a unified standard for the cutoff value, and the inconsistency of the research design of each study reduced the authority of this study.
Conclusions
In short, the results of this meta-analysis show that ADRR has good sensitivity and specificity for PA screening, while the negative likelihood is relatively low and the positive likelihood is relatively high. Therefore, the determination of ADRR is considered to be an effective and convenient PA screening tool.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2272/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2272/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of this research and have worked diligently to confirm the accuracy or completeness of any part, and that related issues are investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rossi GP, Pessina AC, Heagerty AM. Primary aldosteronism: an update on screening, diagnosis and treatment. J Hypertens 2008;26:613-21. [Crossref] [PubMed]
- Käyser SC, Dekkers T, Groenewoud HJ, et al. Study Heterogeneity and Estimation of Prevalence of Primary Aldosteronism: A Systematic Review and Meta-Regression Analysis. J Clin Endocrinol Metab 2016;101:2826-35. [Crossref] [PubMed]
- Xu Z, Yang J, Hu J, et al. Primary Aldosteronism in Patients in China With Recently Detected Hypertension. J Am Coll Cardiol 2020;75:1913-22. [Crossref] [PubMed]
- Funder JW, Carey RM, Mantero F, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016;101:1889-916. [Crossref] [PubMed]
- Monticone S, Burrello J, Tizzani D, et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J Am Coll Cardiol 2017;69:1811-20. [Crossref] [PubMed]
- Okazaki-Hada M, Moriya A, Nagao M, et al. Different pathogenesis of glucose intolerance in two subtypes of primary aldosteronism: Aldosterone-producing adenoma and idiopathic hyperaldosteronism. J Diabetes Investig 2020;11:1511-9. [Crossref] [PubMed]
- Shi S, Lu C, Tian H, et al. Primary Aldosteronism and Bone Metabolism: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2020;11:574151. [Crossref] [PubMed]
- Beard JR, Officer A, de Carvalho IA, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet 2016;387:2145-54. [Crossref] [PubMed]
- Ma L, Song Y, Mei M, et al. Age-Related Cutoffs of Plasma Aldosterone/Renin Concentration for Primary Aldosteronism Screening. Int J Endocrinol 2018;2018:8647026. [Crossref] [PubMed]
- Burrello J, Monticone S, Buffolo F, et al. Diagnostic accuracy of aldosterone and renin measurement by chemiluminescent immunoassay and radioimmunoassay in primary aldosteronism. J Hypertens 2016;34:920-7. [Crossref] [PubMed]
- Tzanela M, Effraimidis G, Vassiliadi D, et al. The aldosterone to renin ratio in the evaluation of patients with incidentally detected adrenal masses. Endocrine 2007;32:136-42. [Crossref] [PubMed]
- Gan W, Lin W, Ouyang J, et al. High efficiency of the aldosterone-to-renin ratio in precisely detecting primary aldosteronism. J Hum Hypertens 2019;33:57-61. [Crossref] [PubMed]
- Luo Q, Li NF, Yao XG, et al. Potential effects of age on screening for primary aldosteronism. J Hum Hypertens 2016;30:53-61. [Crossref] [PubMed]
- Glinicki P, Jeske W, Bednarek-Papierska L, et al. The ratios of aldosterone / plasma renin activity (ARR) versus aldosterone / direct renin concentration (ADRR). J Renin Angiotensin Aldosterone Syst 2015;16:1298-305. [Crossref] [PubMed]
- Lonati C, Bassani N, Gritti A, et al. Measurement of plasma renin concentration instead of plasma renin activity decreases the positive aldosterone-to-renin ratio tests in treated patients with essential hypertension. J Hypertens 2014;32:627-34. [Crossref] [PubMed]
- Rossi GP, Barisa M, Belfiore A, et al. The aldosterone-renin ratio based on the plasma renin activity and the direct renin assay for diagnosing aldosterone-producing adenoma. J Hypertens 2010;28:1892-9. [Crossref] [PubMed]
- Li T, Ma Y, Zhang Y, et al. Feasibility of Screening Primary Aldosteronism by Aldosterone-to-Direct Renin Concentration Ratio Derived from Chemiluminescent Immunoassay Measurement: Diagnostic Accuracy and Cutoff Value. Int J Hypertens 2019;2019:2195796. [Crossref] [PubMed]
- Qin Y, Zhang X, Lou Y, et al. Aldosrerone to direct renin concentration ratio combined with aldosterone concentration for screening primary aldosteronism in elderly hypertensive patients. Chin J Hypertens 2020;28:856-61.
- Teruyama K, Naruse M, Tsuiki M, et al. Novel chemiluminescent immunoassay to measure plasma aldosterone and plasma active renin concentrations for the diagnosis of primary aldosteronism. J Hum Hypertens 2022;36:77-85. [Crossref] [PubMed]
- Morimoto R, Ono Y, Tezuka Y, et al. Rapid Screening of Primary Aldosteronism by a Novel Chemiluminescent Immunoassay. Hypertension 2017;70:334-41. [Crossref] [PubMed]
- Jansen PM, van den Born BJ, Frenkel WJ, et al. Test characteristics of the aldosterone-to-renin ratio as a screening test for primary aldosteronism. J Hypertens 2014;32:115-26. [Crossref] [PubMed]
- Ma WJ, Lou Y, Bian J, et al. Application of aldosterone/direct renin ratio before drug washout in the screening of primary aldosteronism. Zhonghua Yi Xue Za Zhi 2020;100:3250-4. [PubMed]
- Lin W, Li Y, Chen D, et al. High Efficiency and Problems of Chemiluminescence Assay-Detected Aldosterone-To-Renin Ratio in Practical Primary Aldosteronism Screening. Int J Hypertens 2020;2020:3934212. [Crossref] [PubMed]
- Valinsky WC, Touyz RM, Shrier A. Aldosterone and Ion Channels. Vitam Horm 2019;109:105-31. [Crossref] [PubMed]
- Vaidya A, Mulatero P, Baudrand R, et al. The Expanding Spectrum of Primary Aldosteronism: Implications for Diagnosis, Pathogenesis, and Treatment. Endocr Rev 2018;39:1057-88. [Crossref] [PubMed]
- Rossi GP. Prevalence and diagnosis of primary aldosteronism. Curr Hypertens Rep 2010;12:342-8. [Crossref] [PubMed]
- Savard S, Amar L, Plouin PF, et al. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension 2013;62:331-6. [Crossref] [PubMed]
- Rossi GP, Cesari M, Cuspidi C, et al. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension 2013;62:62-9. [Crossref] [PubMed]
- Raizman JE, Diamandis EP, Holmes D, et al. A renin-ssance in primary aldosteronism testing: obstacles and opportunities for screening, diagnosis, and management. Clin Chem 2015;61:1022-7. [Crossref] [PubMed]
(English Language Editor: J. Jones)





