
Bortezomib, epirubicin, and dexamethasone (PAD) results in superior free-progression survival compared to bortezomib, cyclophosphamide, and dexamethasone (VCD) treatment in non-transplantation newly diagnosed multiple myeloma patients aged between 50 to 65: a retrospective single-center analysis in non-transplant patients
Introduction
According to the National Comprehensive Cancer Network (NCCN) Guidelines (Version 1.2020) (1), the first-line therapy for patients with newly diagnosed multiple myeloma (NDMM) is induction with combination bortezomib, lenalidomide, and dexamethasone (VRD) followed by autologous stem cell transplantation (ASCT). However, VRD and ASCT are not readily accessible for the majority of patients in China due to the high costs involved. Consequently, other cytotoxic agents in combination with bortezomib is often used in China (2,3). However, it remains to be determined which combination therapy is most effective for NDMM patients who are eligible for but not receiving ASCT.
According to the latest NCCN recommendation, combined bortezomib, cyclophosphamide, and dexamethasone (VCD) treatment or combined bortezomib, epirubicin, and dexamethasone (PAD) should be used as the primary therapy for transplant candidates. Interestingly, VCD has been shown to result in superior patient survival compared to bortezomib and dexamethasone (VD), VRD, or bortezomib, thalidomide, and dexamethasone (VTD) (4-7), and has been recommended as category 1 therapy. Furthermore, PAD performed noticeably better compared to combined vincristine, doxorubicin, and dexamethasone (VAD) treatment (8). While there has been little data comparing the efficacy of VCD and PAD, some studies have suggested that patients treated with VCD shows similar survival to patients given PAD, however, PAD may result in more adverse events (9-11). Therefore, it is necessary to investigate which regime is more effective for patients enrolled in this study and this current study was performed to determine the optimum therapy for NDMM patients aged 50–65 years old who are eligible for but refuse treatment with ASCT.
Multiple myeloma (MM) is a heterogeneous disease characterized by chromosomal translocation, deletion, and amplification of plasma cells, resulting in a substantial heterogeneity in treatment outcomes (12-14). Amp(1q21), t(4; 14), and del(17p) are commonly observed cytogenetic abnormalities associated with MM and have been shown to be independent prognostic factors of MM (15-17). In addition to the risk stratification developed by the International Myeloma Working Group (IMWG), a new “double-hit” prognostic system was developed based on the co-existence of 2 or more high-risk abnormalities. Early identification facilitates the early prevention and treatment of relapse and progression in patients with NDMM (18,19). This investigation was conducted to identify the optimum therapy for NDMM patients with different cytogenetic backgrounds. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-394/rc).
Methods
Patients and inclusion criteria
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all individual participants included in this study. The study was approved by the Medical Ethics Committee of Chang Zheng Hospital (No. AFHEC012).
A total of 140 NDMM patients with complete clinical records and follow-up data were retrospectively recruited from the Chang Zheng Hospital, Shanghai, China. All patients were newly diagnosed between 21th August, 2011 and 1st December, 2016. The chromosome karyotypes of all recorded patients were determined using fluorescence in situ hybridization (FISH) technology. All routine examinations for MM were conducted prior to treatment. Patients were included in this study if they satisfied the following inclusion criteria: (I) received specific induction therapy, being either VCD or PAD; (II) did not undergo ASCT; (III) presented with specific cytogenetic abnormalities including t(4; 14), del(17p), or amp(1q21) (defined as more than 20% clonal plasma cells positive as detected by FISH; and (IV) had a complete set of clinical data (including basic information, FISH results, and treatment outcomes).
Chemotherapy and maintenance treatment
All patients received induction therapy (median, 4 cycles) until partial remission (PR) or better was achieved. This was followed by 100 mg oral thalidomide every night as the maintenance therapy. As there are currently no existing guidelines, the use of VCD or PAD was at the discretion of the attending physician, in consultation with the patient’s condition and preference. The VCD treatment consisted of 1.3 mg/m2 bortezomib on days 1, 4, 8, and 11; 200 mg cyclophosphamide (i.v.) on days 1–4; and 20 mg dexamethasone (i.v.) on days 1–4 and 7–10 (160 mg/cycle, repeated every 28 days). The PAD regimen consisted of 1.3 mg/m2 bortezomib on days 1, 4, 8, and 11; 50 mg/m2 epirubicin (i.v.) on day 1; and 20 mg dexamethasone (i.v.) on days 1–4 and 7–10 (160 mg/cycle, repeated every 28 days). A total of 11 (7.86%) patients were lost to follow-up, and the median follow-up time was 17.97 months [95% confidence interval (CI): 11.67–27.97 months]. The follow-up period was defined as the time from diagnosis to the first progression or death. All patients were transplant candidates who refused to receive ASCT due to various reasons.
Statistical analysis
Patient characteristics and indexes including age, serum protein electrophoresis, immunoprotein quantification, organ damage, extra-medullary infiltration (time and site), Revised-International Staging System (R-ISS) stage, lactic dehydrogenase (LDH) levels, FISH and flow cytometry data, co-morbidities, therapies, adverse events, and progression free survival (PFS) were collated. All statistical analyses were performed using SPSS18.0 software. Normally distributed data is described as mean ± standard deviations (SD). Abnormally distributed data is described as median and interval quartile range (IQR). The baseline values of all patient indexes were compared using analysis of variance (ANOVA) and non-parametric statistical tests (including Mann-Whitney U test and Bonferroni test). The Kaplan-Meier curve was used to determine the variables which may affect the PFS and the potential variables were analyzed via Cox regression to control for confounding factors.
Results
Patients and baseline characteristics
Due to the significant differences in younger patients (those younger than 50 years old) and older patients (those aged 65 years and older) (20), this study enrolled 140 patients aged between 50 and 65 years old who were eligible for but refused ASCT treatment. Figure 1 shows the flow chart of the selection process. Of the 140 patients, 56 received VCD and 84 received PAD. The baseline characteristics are showed in Table 1. The median age of the VCD group and the PAD group is 61 years (IQR, 57–64 years) and 59 years (IQR, 56–62 years), respectively. For further analysis, the ages of all included patients were classified into three groups, namely, those aged 50–55, 56–60, and 61–65 years. Kaplan-Meier analysis showed statistically significant difference between treatment type (VCD or PAD) (P<0.001), however, there was no significant difference observed with the other indexes (Figure 2A-2H). As age is known to be an important prognostic factor (21), R-ISS staging and age were taken into consideration in the Cox regression to control for confounding factors (22). As patient comorbidities can play a role in the selection of the type of therapy as well as prognosis (23), patient basic characteristics and comorbidities were analyzed (Table 2).

Table 1
| Characteristics | VCD (n=56) | PAD (n=84) | P value |
|---|---|---|---|
| Age, years, median (IQR) | 61 (57.0–64.0) | 59 (560–62.0) | 0.014 |
| Distribution, n (%) | |||
| 50–55 | 11 (19.6) | 20 (23.8) | |
| 56–60 | 14 (25.0) | 34 (40.5) | |
| 61–65 | 31 (55.4) | 30 (35.7) | |
| Gender, n (%) | 0.298 | ||
| Male | 32 (57.1) | 53 (63.1) | |
| Female | 24 (42.9) | 31 (36.9) | |
| Creatinine, µmol/L, median (IQR) | 78.50 (60.00–209.25) | 72.00 (55.50–108.75) | 0.102 |
| Renal insufficiency, n (%) | 9 (36.0) | 14 (23.7) | 0.187 |
| M protein, n (%) | 0.059 | ||
| IgA | 8 (14.3) | 27 (32.1) | |
| IgD | 3 (5.4) | 7 (8.3) | |
| IgG | 28 (50.0) | 34 (40.5) | |
| Nonsecretory | 3 (5.4) | 1(1.2) | |
| Light chain | 12 (21.4) | 15 (17.9) | |
| Missing data | 2 (3.6) | 0 | |
| Serum free light chain, n (%) | 0.409 | ||
| κ | 28 (50.0) | 39 (46.4) | |
| λ | 25 (44.6) | 44 (52.4) | |
| Missing | 3 (5.4) | 1 (1.2) | |
| R-ISS stage, n (%) | 0.730 | ||
| I | 10 (17.9) | 17 (20.2) | |
| II | 33 (58.9) | 52 (61.9) | |
| III | 13 (23.2) | 15 (17.9) | |
| High LDH, n (%) | 11 (20.0) | 13 (15.5) | 0.320 |
| CRP, mg/L, median (IQR) | 3.26 (1.37–8.16) | 4.33 (1.39–6.83) | 0.833 |
| β2MG, mg/L, median (IQR) | 4.70 (2.61–9.82) | 3.63 (2.32–7.50) | 0.164 |
| BMPC, %, median (IQR) | 29.00 (16.00–51.50) | 22.00 (10.88–40.38) | 0.130 |
| Monocyte, %, median (IQR) | 6.85 (5.45–7.80) | 6.10 (4.60–7.10) | 0.061 |
| Extramedullary invasion, n (%) | 9 (16.7) | 15 (18.8) | 0.473 |
| Cytogenetic abnormalities | |||
| t(4; 14) | 4 (7.7) | 19 (26.8) | |
| 17p– | 4 (7.1) | 8 (9.5) | |
| 1q21+ | 35 (62.5) | 47 (56.0) | |
| Recruitment duration | 2013/10–2016/12 | 2011/8–2016/7 | |
| Treatment cycle, median [range] | 4 [1–6] | 4 [1–8] |
a, a total of 140 NDMM patients aged 50–65 who were eligible but refused to receive ASCT. ANOVA test, Mann-Whitney U test, and Bonferroni test were used to analyze the differences between the two groups. The diagnostic criteria and R-ISS staging were in accordance with the International Myeloma Working Group. The positive of cytogenetic abnormalities were defined as more than 20% of clone plasma cell as detected by FISH. VCD, bortezomib, cyclophosphamide and dexamethasone; PAD, bortezomib, epirubicin and dexamethasone; IQR, interquartile range; RISS, revised international myeloma staging system; LDH, lactic dehydrogenase; CRP, C-reactive protein; β2MG, β2-microglobulin; BMPC, bone marrow plasma cell ratio; NDMM, newly diagnosed multiple myeloma; ASCT, autologous stem cell transplantation; ANOVA, analysis of variance.
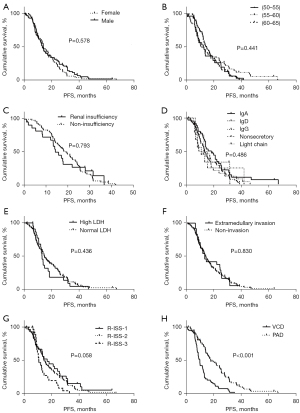
Table 2
| Comorbidities | VCD (n=56) | PAD (n=84) | P value |
|---|---|---|---|
| Hypertension, n (%) | 22 (44.6) | 22 (26.2) | 0.102 |
| Diabetes, n (%) | 7 (12.5) | 3 (3.6) | 0.094 |
| Coronary heart disease, n (%) | 5 (8.9) | 2 (2.4) | 0.178 |
| Hepatic disease, n (%) | 4 (7.1) | 8 (9.5) | 0.820 |
#, there was a total of 140 NDMM patients aging 50 to 65 who were eligible but refused to receive ASCT therapy. The Mann-Whitney U test and Bonferroni test were used to analyze the differences between the two groups. The diagnosis of hypertension was in accordance with the criteria of the ESC and the AHA. The diagnosis of diabetes was based on the criteria of the ADA. The diagnosis of coronary heart disease referred to the criteria of the ACC and the ESC. Hepatic disease included hepatitis and cirrhosis and the diagnostic criteria was in consultation with the guidelines of the CMA. VCD, bortezomib, cyclophosphamide, and dexamethasone; PAD, bortezomib, epirubicin, and dexamethasone; ESC, European Society of Cardiology; AHA, American Heart Association; ADA, American Diabetes Association; ACC, American College of Cardiology; ESC, European Society of Cardiology; CMA, Chinese Society of Hepatology; NDMM, newly diagnosed multiple myeloma; ASCT, autologous stem cell transplantation;
Efficiency
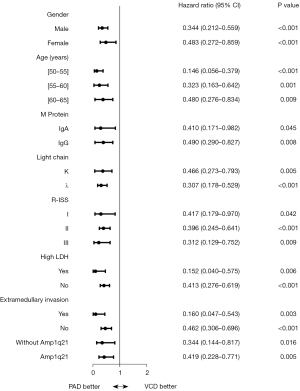
Analysis of the 140 patients demonstrated that patients treated with PAD had longer PFS and a superior response rate compared to patients treated with VCD. There was a significant difference in the age between patients in the VCD treatment group and those in the PAD group (P=0.014). When the age variable was converted to a categorical variable, Kaplan-Meier analysis showed no significant association between age and PFS (P=0.441). Furthermore, when age was considered a classified variable, Cox regression analysis revealed that there was no statistical significance in age between the two treatment groups. PFS was 7.9 months longer in patients treated with PAD (17.97 months; range, 10.83–28.90 months) compared to patients treated with VCD (10.07 months; range, 7.30–13.43 months) (P<0.001), with a hazard ratio of 0.385 (0.265–0.558). Moreover, patients treated with PAD showed a more satisfactory response rate (47/56, 83.9%) compared to patients treated with PAD (77/84, 91.7%) (P=0.087; Table 3). These data demonstrated that, compared to VCD, PAD treatment resulted in superior PFS and response rate in NDMM patients aged 50 to 65 years who are eligible for but not receiving ASCT.
Table 3
| Effect | VCD (n=56) | PAD (n=84) |
|---|---|---|
| Post-induction effect, n (%) | ||
| CR | 14 (25.0) | 30 (35.7) |
| PR | 24 (42.9) | 39 (46.4) |
| VGPR | 10 (17.9) | 19 (22.6) |
| VGPR or better | 47 (83.9) | 77 (91.7) |
| SD | 7 (12.5) | 5 (6.0) |
| PD | 2 (3.6) | 1 (1.2) |
| Missing | 0 (0) | 1 (1.2) |
| PFS, months, median (IQR) | 10.07 (7.30–13.43) | 17.97 (10.83–28.90) |
#, there was a total of 140 patients who were treated with VCD or PAD. Descriptive statistical analysis was used to assess the distribution of PFS. Response or progression were defined according to the International Myeloma Working Group criteria. VCD, bortezomib, cyclophosphamide, and dexamethasone; PAD, bortezomib, epirubicin, and dexamethasone; CR, complete response; PR, partial response; VGPR, very good partial response; SD, stable disease; PD, progressive disease; PFS, progression-free survival; IQR, interquartile range.
PAD treatment also resulted in better PFS and response rates in all cytogenetic abnormality groups compared to VCD treatment. The cytogenetic background of all 140 patients was analyzed and 114 patients with the cytogenetic abnormalities amp (1q21), del(17p), or t(4; 14), were selected for further investigation. Patients in all cytogenetic abnormality groups showed superior median PFS after treatment with PAD compared to patients treated with VCD, except for patients with the t(4; 14) abnormality, where the variations in the sample size prevented an accurate comparison (3 patients received VCD and 17 received PAD). The outcomes for the other subgroups are shown in Figure 3.
Discussion
To the best of our knowledge, this single-center retrospective analysis is the first to demonstrated that, compared to VCD treatment, PAD treatment resulted in a longer PFS and a superior response rate in NDMM patients aged 50 to 65 years old who were eligible for but not receiving ASCT therapy.
There is limited literature comparing the efficacy of VCD and PAD in NDMM patients. Contrary to our current investigation, Mai et al. (9) suggested that VCD may be a more advantageous induction therapy compared to PAD in patients receiving ASCT therapy. However, there are numerous differences between the 2 studies which may explain the seemingly contrasting results.
The first and foremost is that the study by Mai et al. (9) is designed as a randomized controlled trial (RCT), while this current study is a retrospective analysis. RCTs are routinely undertaken in specific trial centers and the outcomes may not reflect real-world conditions. By contrast, a retrospective study is much more representative of the real world as the patients are all selected from the real-world but not pre-designed. Indeed, many studies have reported different results between RCTs and real-world conditions (24-27). The second difference is in the doses of cyclophosphamide and dexamethasone. While cyclophosphamide was used at a dose of 900 mg/m2 by Mai et al. (9), the dose used in our study was 800 mg in accordance with the guidelines for diagnosis and treatment of multiple myeloma in China. In addition, Mai and colleagues used a high-dose of dexamethasone (9). Rajkumar’s study has suggested that low-dose dexamethasone may have a more satisfactory PFS and lower toxicity compared with high-dose dexamethasone in NDMM patients (28). In addition, differences in the race and age of the study cohort may contribute to differences in the incidence of MM and patient outcomes (29-31). Finally, Mai’s study suggested that VCD maybe an advantageous induction therapy prior to receiving ASCT treatment (9). However, the patients in our analysis received VCD or PAD as chemotherapy treatment rather than induction therapy. Therefore, the terminal point of Mai’s study was to receive ASCT therapy (9), whereas, the endpoint for our patients was remission using VCD or PAD therapy.
This current report has particular significance for NDMM patients in China. First, as few as 20% of patients are eligible for and will undergo ASCT therapy in China. This is largely due to the associated side effects and massive costs associated with ASCT and VRD therapy. In fact, in Shanghai, an estimated 23% of patients are eligible, and only 21% will actually undergo ASCT. By contrast, VCD and PAD therapy is more accessible but it remains controversial which is the better treatment regimen (32). The current investigation demonstrated that PAD is superior both in terms of longer PFS and a better response rate in NDMM patients aged 50 to 65 years old who were eligible for but not receiving ASCT therapy. Second, MM is generally a condition which affects people over the age of 50 years (33). Consistent with this, in our study cohort, 16% (100/625) of patients diagnosed with NDMM were less than 50 years old. Patients aged 50 to 65 years account for approximately 10–20% of all NDMM patients in China. Therefore, developing the optimum therapy for this age group will be applicable to a significant portion of MM patients in China. Third, this study demonstrated that treatment outcomes were not affected by differences in cytogenetic abnormalities in the PAD group nor the VCD treatment group. Indeed, bortezomib-based induction therapy could partially improve poor prognosis in all patients. Noteworthy is that most of the patients with cytogenetic abnormalities were likely to change therapy or were treated with more than three agents [group B: 78/253 (69.17%) and group C: 71/99 (71.72%)], and this may have contributed to the outcomes.
In conclusion, this retrospective analysis demonstrated that, compared to VCD, PAD treatment resulted in a longer PFS in NDMM patients aged 50–65 years old who were eligible for but did not receive ASCT therapy in China. There are some limitations to this study. First, in order to prevent severe adverse events and toxicities in elderly or frail patients, reduced-intensity induction therapy is used in those patients. Therefore, the regime and dose in this study may not apply to them. In fact, the adverse events are also important considerations in the choice of therapy. However, may be due to the small sample size of this study, only common side effects such as nausea, fatigue, diarrhea, headache are observed, while no severe side effects are observed. Second, this was a single-center study and there may be some coincidences and systematic mistakes. Finally, while the retrospective nature of this study means it is more representative of the real-world situation, there are certain factors that could not be controlled for and these may result in bias of outcomes. Future studies will examine the underlying mechanisms giving rise to the differences observed with the two treatment regimes.
Acknowledgments
Funding: The authors would like to acknowledge funding from the National Nature Science Foundation of China (No. 81870159); the Talent Project of Pujiang, Shanghai (No. 18PJD059); the Outstanding Youth Reserve of Pyramid Talent Project and the Research oriented Physician Developing Program in the Second Affiliated Hospital of Navy Military Medical University.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist Available at https://atm.amegroups.com/article/view/10.21037/atm-22-394/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-394/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-394/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all individual participants included in this study. The study was approved by the Medical Ethics Committee of Chang Zheng Hospital (No. AFHEC012).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kumar SK, Callander NS, Hillengass J, et al. NCCN Guidelines Insights: Multiple Myeloma, Version 1.2020. J Natl Compr Canc Netw 2019;17:1154-65. [Crossref] [PubMed]
- Fiala MA, Wildes TM. Racial disparities in treatment use for multiple myeloma. Cancer 2017;123:1590-6. [Crossref] [PubMed]
- Costa LJ, Huang JX, Hari PN. Disparities in utilization of autologous hematopoietic cell transplantation for treatment of multiple myeloma. Biol Blood Marrow Transplant 2015;21:701-6. [Crossref] [PubMed]
- Reeder CB, Reece DE, Kukreti V, et al. Long-term survival with cyclophosphamide, bortezomib and dexamethasone induction therapy in patients with newly diagnosed multiple myeloma. Br J Haematol 2014;167:563-5. [Crossref] [PubMed]
- Reeder CB, Reece DE, Kukreti V, et al. Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood 2010;115:3416-7. [Crossref] [PubMed]
- Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012;119:4375-82. [Crossref] [PubMed]
- Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia 2009;23:1337-41. [Crossref] [PubMed]
- Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol 2012;30:2946-55. [Crossref] [PubMed]
- Mai EK, Bertsch U, Dürig J, et al. Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAD) in newly diagnosed myeloma. Leukemia 2015;29:1721-9. [Crossref] [PubMed]
- Yagi H. Initial treatment strategy for patients newly diagnosed with multiple myeloma. Rinsho Ketsueki 2017;58:2050-7. [PubMed]
- He J, Yang L, Han X, et al. The choice of regimens based on bortezomib for patients with newly diagnosed multiple myeloma. PLoS One 2014;9:e99174. [Crossref] [PubMed]
- Hanamura I. Gain/Amplification of Chromosome Arm 1q21 in Multiple Myeloma. Cancers (Basel) 2021;13:256. [Crossref] [PubMed]
- Castaneda O, Baz R. Multiple Myeloma Genomics - A Concise Review. Acta Med Acad 2019;48:57-67. [Crossref] [PubMed]
- Perrot A, Lauwers-Cances V, Tournay E, et al. Development and Validation of a Cytogenetic Prognostic Index Predicting Survival in Multiple Myeloma. J Clin Oncol 2019;37:1657-65. [Crossref] [PubMed]
- Padala SA, Barsouk A, Barsouk A, et al. Epidemiology, Staging, and Management of Multiple Myeloma. Med Sci (Basel) 2021;9:3. [Crossref] [PubMed]
- Qian J, Jin J, Luo H, et al. Analysis of clinical characteristics and prognostic factors of multiple myeloma: a retrospective single-center study of 787 cases. Hematology 2017;22:472-6. [Crossref] [PubMed]
- Shah V, Sherborne AL, Walker BA, et al. Prediction of outcome in newly diagnosed myeloma: a meta-analysis of the molecular profiles of 1905 trial patients. Leukemia 2018;32:102-10. [Crossref] [PubMed]
- Ashby C, Tytarenko RG, Wang Y, et al. Poor overall survival in hyperhaploid multiple myeloma is defined by double-hit bi-allelic inactivation of TP53. Oncotarget 2019;10:732-7. [Crossref] [PubMed]
- Walker BA, Morgan GJ. The genomic features associated with high-risk multiple myeloma. Oncotarget 2018;9:35478-9. [Crossref] [PubMed]
- Palumbo A, Bringhen S, Mateos MV, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood 2015;125:2068-74. [Crossref] [PubMed]
- Puyade M, Defossez G, Guilhot F, et al. Age-related health care disparities in multiple myeloma. Hematol Oncol 2018;36:224-31. [Crossref] [PubMed]
- Stukel TA, Fisher ES, Wennberg DE, et al. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA 2007;297:278-85. [Crossref] [PubMed]
- Song X, Cong Z, Wilson K. Real-world treatment patterns, comorbidities, and disease-related complications in patients with multiple myeloma in the United States. Curr Med Res Opin 2016;32:95-103. [Crossref] [PubMed]
- Bergin K, McQuilten Z, Moore E, et al. Myeloma in the Real World: What Is Really Happening? Clin Lymphoma Myeloma Leuk 2017;17:133-144.e1. [Crossref] [PubMed]
- Gray E, Norris S, Schmitz S, et al. Do disparities between populations in randomized controlled trials and the real world lead to differences in outcomes? J Comp Eff Res 2017;6:65-82. [Crossref] [PubMed]
- Richardson PG, San Miguel JF, Moreau P, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J 2018;8:109. [Crossref] [PubMed]
- Jagannath S, Roy A, Kish J, et al. Real-world treatment patterns and associated progression-free survival in relapsed/refractory multiple myeloma among US community oncology practices. Expert Rev Hematol 2016;9:707-17. [Crossref] [PubMed]
- Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol 2010;11:29-37. [Crossref] [PubMed]
- Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population-based study. Blood 2010;116:5501-6. [Crossref] [PubMed]
- Ailawadhi S, Bhatia K, Aulakh S, et al. Equal Treatment and Outcomes for Everyone with Multiple Myeloma: Are We There Yet? Curr Hematol Malig Rep 2017;12:309-16. [Crossref] [PubMed]
- Huang BT, Tan Y, Zhao WH, et al. How to determine bortezomib-based regimen for elderly patients with multiple myeloma: PAD versus CBd, an observational study. J Cancer Res Clin Oncol 2014;140:303-9. [Crossref] [PubMed]
- Shi H, Chen Z, Xie J, et al. The Prevalence and Management of Multiple Myeloma-Induced Kidney Disease in China. Kidney Dis (Basel) 2016;1:235-40. [Crossref] [PubMed]
- Gerecke C, Fuhrmann S, Strifler S, et al. The Diagnosis and Treatment of Multiple Myeloma. Dtsch Arztebl Int 2016;113:470-6. [Crossref] [PubMed]


