
Improvement of surgical techniques for orthotopic single lung transplantation in rats
Introduction
Lung transplantation is the ultimate treatment option for a variety of end-staged lung diseases (1). However, the survival rate of lung transplantation is greatly inferior to that of other solid organ transplantations (2). Animal models, especially rodent transplant models, are critical to better understand the complex molecular and cellular mechanism of lung transplantation and improve the clinical outcome (3). A rat transplant model was first established by Asimacopoulos et al. in 1971 (4). Later in 1989, the cuff techniques were introduced by Mizuta et al. (5), which dramatically facilitated anastomoses of the pulmonary artery and vein. Although a variety of modifications of surgical and cuff techniques have since been established (6-8), the rat transplantation model is still highly demanding and can only be performed successfully in select laboratories (9-12).
The most challenging part of rat lung transplantation with cuff technique lies in the anastomoses of the pulmonary artery and vein between the donor and recipient, which may raise many difficulties for successful reperfusion. These difficulties arise because of unclear surgical fields, misuses of microvascular clip and improper angles. Previous reports have shown that one of the major reasons for failure of this model is twisting or laceration of vessels caused by an improper angle of cuff insertion (7,13). Since the pulmonary vein is much shorter and more fragile than the pulmonary artery, it is rather difficult to achieve the optimal angle to insert the donor’s cuff into the recipient’s vein. In certain cases, the pulmonary vein is divided into two branches, which further narrows the space for the procedure. Thus, a longer pulmonary vein can help facilitate the anastomoses and reduce the probability of vessel twist and laceration. Pulmonary vein embolism and airway complications are another two common reasons for the death (14). Therefore, novel surgical techniques to overcome these shortcomings are needed to further improve the survival rate of animal models.
In the present study, we described our improvements in anesthesia, lung extraction, vascular clamp, and transplantation procedures, which can shorten anastomoses time, reduce vascular complications, and improve survival rate in a sample size as large as possible. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2018/rc).
Methods
Animals
Eight-week-old male inbred Sprague Dawley (SD) rats weighing 250–300 g were used as both donors and recipients, and were purchased from Charles River Laboratories (Beijing, China). A total of 100 donor rats and an equal number of recipient rats were equally randomized into conventional technique and improved technique groups, using a computer based random order generator. Anastomoses time, vascular complications, and survival rate at post-operative day 7 were compared between the two groups. All rats were housed under pathogen-free conditions at Tongji University Animal Facility (Shanghai, China), with free access to water and laboratory food. A protocol was prepared before the study without registration. All animal experiments were approved by the Animal Care and Use Committee of Shanghai Pulmonary Hospital (No. K21-282, Shanghai, China), in compliance with the Guide for the Care and Use of Laboratory Animals, 8th edition.
Traditional procedure
Rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg, Sinopharm, Beijing, China), intubated with a 14-G catheter via tracheostomy, and mechanically ventilated with ambient air at a tide volume of 10 mL/kg, and a respiratory rate of 80/minute (KW-100-2, KEW Basis, Beijing, China). A heating pad at 32 ℃ was used to maintain the temperature. All procedures were performed using a surgical microscope with 10× magnification (XTS-4A, Zhongtian Optics, Zhenjiang, China).
The donor rat was placed in the supine position, and a median laparosternotomy was performed. After removing diaphragms, 100 units of heparin (Sinopharm, Beijing, China) were administered via the inferior vena cava. Then, the thymus was excised to expose the heart and lungs. The left auricle was cut and a 22-G catheter was introduced into the pulmonary trunk. The lungs were perfused with 20 mL of cold fluid (4 ℃) of low potassium dextran glucose (Perfadex; Medisan, Uppsala, Sweden). Then, the heart and lungs were en bloc harvested at end-tide volume and placed on ice. Subsequently, the left lung was isolated, with left bronchus, pulmonary artery and vein dissected for further cuffs placement. The cuffs with a tail for bronchus, pulmonary vein and artery are made of 14-G, 16-G, and 18-G catheters, respectively. The bronchus and vessels were passed through the cuffs, with the proximal end everted over the cuff and then fixed with a circumferential ligature of 5-0 silk suture (Figure 1A). The lung was placed on ice and wrapped by sterile gauze before transplantation.
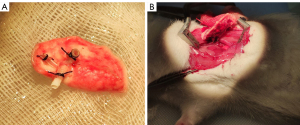
The recipient rats were anesthetized, intubated, and ventilated in the same manner as donors. They were placed in the right lateral decubitus position and left thoracotomy was carried out through the fourth intercostal space. The left lung was pulled out from the cavity by a blood vessel clamp and maintained proper tension to expose the hilum. The pulmonary bronchus, artery, and vein were isolated, and clamped by micro-vascular clamps, separately. Pre-knotted 5-0 silk ligatures were placed around the left main bronchus. An incision was made on the anterior wall of distal bronchus, into which the bronchus cuff of the donor lung was inserted and fixed with the silk ligature. The same procedure was repeated for the vein and artery. After that, the left lung was resected and the clamp was removed to initiate reperfusion (Figure 1B). Finally, the chest cavity, subcutis, and skin were closed with 5-0 Vicryl suture (Ethicon Inc., Johnson and Johnson, Raritan, NJ, USA). The recipient rats were then extubated.
Improvement of surgical techniques
Based on the traditional procedures, we made several modifications, as follows:
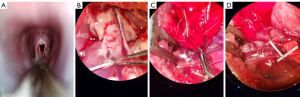
- Orotracheal intubation instead of tracheostomy was performed via a video laryngoscope for the rats (Figure 2A);
- After anterograde perfusion, the pulmonary trunk was cut and retrograde perfusion with 10 mL cold fluid (4 ℃) of low potassium dextran glucose (Perfadex) was performed through the left auricle (Figure 2B);
- Instead of using conventional microvascular clamp, a Rummel tourniquet for the rat was made with a 22-G intravenous catheter to temporarily occlude the pulmonary artery and vein (Figure 2C,2D);
- After incisions were made in the recipient’s pulmonary artery and vein, both the vessels and the cuffs were flushed with heparin;
- A 20-G catheter was inserted into the chest cavity for pleural drainage before the thorax was closed. The catheter could be connected to a syringe for aspirating to circumvent pneumothorax.
Outcome assessments
The warm ischemia time was defined as the time between perfusion with low potassium dextran glucose and lung removal from hypothermia storage. The cold ischemia time was defined as the time between the donor lung removal from ice storage and reperfusion, namely the anastomosis time. The donor and recipient operation time were defined as the time from intubation to heart-lung retrieval and closure of skin, respectively.
On post-operative day 7, the survival rate of recipient rats was calculated and the surviving rats were sacrificed. Anesthesia, intubation, and mechanical ventilation were carried out. Then the transplanted left lungs and normal right lungs were harvested, fixed in 4% paraformaldehyde, sectioned, and stained with hematoxylin and eosin (HE) for histology analysis. Grading for infiltration pathology was performed by three pathologists independently using standard criteria reported by the American Thoracic Society Workshop (15). To be brief, the lung infiltration grading system, consists of edema, alveolar congestion, hemorrhage and aggregation of neutrophils in alveolar cavity or vascular wall, which was scored in a 0-to-4 scale (0 refers to normal lung tissue; 1 refers to light infiltration; 2 refers to moderate infiltration; 3 refers to strong infiltration; 4 refers to severe infiltration). The average of scores of these four domains were used to represent the final score of the rat lung infiltration.
Statistical analysis
Categorical variables were summarized in frequency and percentage, while continuous variables were presented in mean ± SD. Fisher’s exact test and Student’s t-test were performed to compare categorical data and continuous data respectively. The software SPSS 26.0 (IBM Corp., Armonk, NY, USA) was used for data analysis and a two-sided P value less than 0.05 was considered statistically significant.
Results
Surgical outcomes
A total of 50 lung transplantations were successfully performed in the traditional procedures group and improvement of surgical techniques group, respectively. The surgical time of donor operation, cuffs preparation, and cold ischemia time were similar between the two groups (Table 1). However, the improvement of surgical techniques significantly reduced the recipient operation time (35.7 vs. 46.3 min, P<0.01) and warm ischemia time (16.3 vs. 28.8 min, P<0.01).
Table 1
| Variables | Traditional procedures (n=50) | Improvement of surgical techniques (n=50) | P |
|---|---|---|---|
| Donor operation time (min) | 17.8±2.6 | 19.3±1.5 | 0.26 |
| Cuffs preparation time (min) | 9.6±3.4 | 8.7±2.3 | 0.56 |
| Recipient operation time (min) | 46.3±4.7 | 35.7±3.3 | <0.01* |
| Cold ischemia time (min) | 32.7±5.8 | 34.2±4.8 | 0.77 |
| Warm ischemia time (min) | 28.8±3.2 | 16.3±4.1 | <0.01* |
| Vascular complications | 0.04* | ||
| No complications | 42 | 49 | |
| Venous laceration/thrombosis | 8 | 1 | |
| Survival rate | 80% | 96% | 0.03* |
Categorical variables were summarized in frequency and percentage, while continuous variables were presented in mean ± SD. *, ‘Improvement of surgical techniques’ versus ‘PGD Grade 0–2’ P<0.05.
Vascular complications and survival rate
In the traditional procedures group, 3 rats died intraoperatively due to pulmonary vein laceration and 5 rats died within seven days postoperatively due to pulmonary vein thrombosis. While in the improvement of surgical techniques group, only 1 rat died intraoperatively due to pulmonary vein laceration and death related to pulmonary vein thrombosis was observed. The vascular complications were significantly higher in the traditional procedures group (16% vs. 2%, P=0.04). Additionally, 2 rats in the traditional procedures group died of pneumothorax and pleural effusion, and 1 rat in the improvement of surgical techniques group died of bronchial obstruction postoperatively. Overall, the survival rate on postoperative day 7 was lower in the traditional procedures group (80% vs. 96%, P=0.03), compared with the improvement of surgical techniques group. The overall survival curve was significantly different from the improved technique group and the conventional technique group (Figure 3).
Histological changes
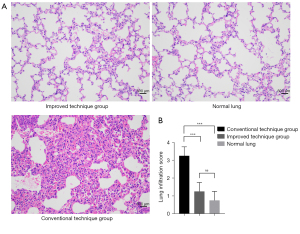
Seven days after transplantation, light microscopy revealed that there were marked inflammatory infiltration and edema in transplanted lung tissues of conventional technique group compared with those in improved technique group or normal lung tissues in contralateral sides. In contrast, no obvious evidence of cellular infiltration was observed in the left grafts of improved technique group compared with contralateral normal lungs (Figure 4).
Discussion
The rat orthotopic lung transplantation model is an important tool to investigate ischemia reperfusion and graft rejection; however, the procedure has remained challenging, with high failure rate. Despite some surgical improvements in rat lung transplantation have previously been reported in the literature (7,8), we are the first group to focus on how might more stable and more widely available anastomoses be achieved. By utilizing the approach reported in this article, the difficulty of the anastomoses of the pulmonary artery and vein can be reduced greatly, which were once thought to be the most challenging part. In the present study, we described several modifications in surgical techniques, and by comparing this technique with conventional procedure, we demonstrated that our improvement in surgical technique can shorten anastomoses time, reduce the vascular complications, and improve survival rate.
Tracheostomy is widely used for intubation in rat models (9), as it is both reliable and simple. However, it is also traumatic, especially for rats requiring long-term survival, such as the transplantation model for chronic rejection. Also, tracheostomy may cause stenosis in trachea and confound the interpretation of pulmonary function. Although orotracheal intubation is less traumatic, it is more technically demanding, since the glottis of the rat is usually obscure. By using a video laryngoscope for the rats, we found the glottis clearly visible, making the orotracheal intubation easily to perform.
The latest research conducted by Lee et al. (16) advocates using a weight-based guide to choose appropriate sizes of artery, vein, and bronchus cuffs. However, our attempt during the transplantation indicated that this approach would not be feasible, because we observed that the diameter of a pulmonary artery and pulmonary vein are in fact not related to rat body weight. To some extent, more weight usually means more fat, and sometimes, finer vasculature.
Pulmonary vein thrombosis is a major cause of death for rat lung transplantation postoperatively. In the present study, we performed both anterograde and retrograde perfusion and flushed the vessels and the cuffs with heparin before transplantation in the modified rat model. Our study revealed that these modifications could significantly reduce the rate of pulmonary vein thrombosis. In fact, additional retrograde perfusion following anterograde perfusion is widely used in clinical practice for human lung transplantation (17). More blood, clots, and fat emboli may be flushed out by retrograde perfusion (18), thus reducing the risk of pulmonary vein thrombosis after transplantation. Previous reports have demonstrated that late retrograde perfusion also contributes to better graft function (19,20), compared with conventional anterograde perfusion. Therefore, retrograde perfusion can be also applied to the rat model to improve the survival rate and better mimic clinical practice.
The anastomosis of pulmonary vein using cuff technique is much more difficult than that of pulmonary artery, due to the friability and short length of pulmonary vein. Several methods have been adopted to increase the length of pulmonary vein in previous studies. Zampieri et al. (13) reported that they placed the vein clamp as close as possible to the left atrium to lengthen the pulmonary vein; however, doing so may also clamp the left superior vena cava, causing cardiac arrhythmia and arrest. Goto et al. used the donor’s descending aorta as interposition grafts for the cuffs, which increased the length of vessels and facilitated the anastomosis (7). Slip knots instead of microvascular clamp have been commonly used to occlude the pulmonary vessels in the mouse model of lung transplantation (21), since the pulmonary vein is even shorter. However, the pulmonary artery and vein of rats are much thicker, and cannot be occluded merely by slip knots. Therefore, in the present study, inspired by the Rummel tourniquet used for transient pulmonary artery occlusion in video-assisted thoracoscopic surgery (22), a small Rummel for the rat was fashioned with 7-0 silk sutures and 22-G catheter to occlude the pulmonary vessels. This modification of the vascular clamp can help to obtain a longer pulmonary vein and better exposure to perform anastomosis. By comparing this technique with conventional vascular clamp, we demonstrated that it can reduce the risk of vein laceration and the anastomosis time. The Rummel tourniquet was also used for hilar occlusion in a murine model of left lung ischemia reperfusion injury (23).
Whether chest drainage is necessary has remained controversial in previous reports (8,24). However, postoperative pneumothorax and pleural effusion are major risk factors of death after successful transplantation. To avoid these severe complications, we inserted a 20-G catheter into the chest cavity for pleural drainage before the thorax was closed in the modification group. The results showed that no rat died due to pneumothorax and pleural effusion in the improvement of surgical techniques group. Therefore, a simple chest drainage should be carried out to further improve the survival rate.
Conclusions
We made several modifications in surgical techniques for orthotopic single lung transplantation in rats. Our improvement of surgical techniques could shorten anastomoses time, reduce the vascular complications, and improve survival rate. Therefore, the improvement will lay a good foundation for the study of the mechanism of human lung transplantation related diseases.
Acknowledgments
Funding: This study was supported by the Scientific and Technological Innovation Action Plan of Science and Technology Commission of Shanghai Municipality (No. 20DZ2253700).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2018/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2018/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2018/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were approved by the Animal Care and Use Committee of Shanghai Pulmonary Hospital (No. K21-282, Shanghai, China), in compliance with the Guide for the Care and Use of Laboratory Animals, 8th edition.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Young KA, Dilling DF. The Future of Lung Transplantation. Chest 2019;155:465-73. [Crossref] [PubMed]
- Chambers DC, Zuckermann A, Cherikh WS, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: 37th adult lung transplantation report - 2020; focus on deceased donor characteristics. J Heart Lung Transplant 2020;39:1016-27. [Crossref] [PubMed]
- Lama VN, Belperio JA, Christie JD, et al. Models of Lung Transplant Research: a consensus statement from the National Heart, Lung, and Blood Institute workshop. JCI Insight 2017;2:e93121. [Crossref] [PubMed]
- Asimacopoulos PJ, Molokhia FA, Pegg CA, et al. Lung transplantation in the rat. Transplant Proc 1971;3:583-5. [PubMed]
- Mizuta T, Kawaguchi A, Nakahara K, et al. Simplified rat lung transplantation using a cuff technique. J Thorac Cardiovasc Surg 1989;97:578-81. [Crossref] [PubMed]
- Zhai W, Ge J, Inci I, et al. Simplified rat lung transplantation by using a modified cuff technique. J Invest Surg 2008;21:33-7. [Crossref] [PubMed]
- Goto T, Kohno M, Anraku M, et al. Simplified rat lung transplantation using a new cuff technique. Ann Thorac Surg 2012;93:2078-80. [Crossref] [PubMed]
- Tian D, Shiiya H, Sato M, et al. Rat lung transplantation model: modifications of the cuff technique. Ann Transl Med 2020;8:407. [Crossref] [PubMed]
- Kubisa B, Schmid RA, Grodzki T. Model of single left rat lung transplantation. Relation between surgical experience and outcomes. Rocz Akad Med Bialymst 2003;48:70-3. [PubMed]
- Habertheuer A, Kocher A, Laufer G, et al. Innovative, simplified orthotopic lung transplantation in rats. J Surg Res 2013;185:419-25. [Crossref] [PubMed]
- Mizobuchi T, Sekine Y, Yasufuku K, et al. Comparison of surgical procedures for vascular and airway anastomoses that utilize a modified non-suture external cuff technique for experimental lung transplantation in rats. J Heart Lung Transplant 2004;23:889-93. [Crossref] [PubMed]
- Marck KW, Wildevuur CR. Lung transplantation in the rat: I. Technique and survival. Ann Thorac Surg 1982;34:74-80. [Crossref] [PubMed]
- Zampieri D, Azzollini N, Vuljan S, et al. Vein Suturing Results in Worse Lung Graft Outcomes Compared to the Cuff Method. Eur Surg Res 2019;60:106-16. [Crossref] [PubMed]
- Jungraithmayr WM, Korom S, Hillinger S, et al. A mouse model of orthotopic, single-lung transplantation. J Thorac Cardiovasc Surg 2009;137:486-91. [Crossref] [PubMed]
- Matute-Bello G, Downey G, Moore BB, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 2011;44:725-38. [Crossref] [PubMed]
- Lee YG, Kim JL, Palmer AF, et al. A Rat Lung Transplantation Model of Warm Ischemia/Reperfusion Injury: Optimizations to Improve Outcomes. J Vis Exp 2021; [Crossref] [PubMed]
- Venuta F, Rendina EA, Bufi M, et al. Preimplantation retrograde pneumoplegia in clinical lung transplantation. J Thorac Cardiovasc Surg 1999;118:107-14. [Crossref] [PubMed]
- Irie M, Otani S, Kurosaki T, et al. Warm retrograde perfusion can remove more fat from lung grafts with fat embolism in a porcine model. Gen Thorac Cardiovasc Surg 2020;68:363-9. [Crossref] [PubMed]
- Wittwer T, Franke UF, Fehrenbach A, et al. Experimental lung transplantation: impact of preservation solution and route of delivery. J Heart Lung Transplant 2005;24:1081-90. [Crossref] [PubMed]
- Strüber M, Hohlfeld JM, Kofidis T, et al. Surfactant function in lung transplantation after 24 hours of ischemia: advantage of retrograde flush perfusion for preservation. J Thorac Cardiovasc Surg 2002;123:98-103. [Crossref] [PubMed]
- Okazaki M, Krupnick AS, Kornfeld CG, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant 2007;7:1672-9. [Crossref] [PubMed]
- Jiang L, Wu L, Roque SR, et al. A novel tourniquet technique for transient pulmonary artery occlusion during video-assisted thoracoscopic surgery. J Thorac Cardiovasc Surg 2018;156:816-8. [Crossref] [PubMed]
- Charles EJ, Chordia MD, Zhao Y, et al. SPECT imaging of lung ischemia-reperfusion injury using [99mTc]cFLFLF for molecular targeting of formyl peptide receptor 1. Am J Physiol Lung Cell Mol Physiol 2020;318:L304-L313. [Crossref] [PubMed]
- Santana Rodríguez N, Llontop Santisteban P, López García A, et al. Technical modifications of the orthotopic lung transplantation model in rats with brain-dead donors. Arch Bronconeumol 2011;47:488-94. [Crossref] [PubMed]
(English Language Editor: J. Jones)



