
Establishment and evaluation of cell and animal models expressing BORIS subfamily 2 variant
Introduction
Brother of the Regulator of Imprinted Sites (BORIS), also called CCCTC-binding Factor Like (CTCFL), is identified as a paralog of CCCTC-binding factor (CTCF) with 11 zinc-finger (ZF) DNA-binding domains (1,2). BORIS belongs to the cancer-testis antigen (CTA) family and is characterized by exclusive expression in nonmalignant tissue with the exception of germ cells (3-5). However, it has been detected as aberrantly highly expressed in numerous types of human cancers, including glioblastoma (6), breast cancer (7,8), esophageal cancer (9), uterine corpus cancer (10,11), and ovarian cancer (12). More importantly, high expression of BORIS plays an important biological role in maintaining stemness of tumor cells (13,14). It has been reported that BORIS is associated with the expression of the cancer stem-like cell (CSC) marker CD90 in hepatocellular carcinoma (HCC) (15). Further research found that BORIS promotes CSC-like traits, including self-renewal, migration and invasion of HCC cells through the epigenetic regulation of OCT4 expression, which is critical to maintain and induce pluripotency stem cells (16). Hence, the CT antigen BORIS is promising as an immunotherapeutic target for a cancer vaccine design. BORIS has already proved to be an effective immunotherapeutic target of breast cancer in murine breast cancer models (17). At present, immunotherapy targeting BORIS has entered a phase I trial for breast cancer treatment (18,19). BORIS contains three promoters and is expressed as 23 mRNA isoforms generated mainly from selective usage of alternative promoters (A, B, and C) and splice sites (20). Based on various numbers of ZF domains and alternative combinations of amino- and carboxyl-terminal ends, BORIS can be subdivided into six subfamilies (sf1 to sf6) (20). The mRNA isoforms A4 and C2, which are classified as sf2, both encode the same polypeptide (20).
HCC is rated as the second deadliest malignant tumor globally due to its resistance to chemotherapy and high rate of recurrence. HCC is characterized by insidious pathogenesis and high metastasis and mortality rates (21,22). Conventional treatments for HCC, including surgery, local or radiofrequency ablation, systemic chemotherapy, and targeted therapy, are suboptimal due to their limitations and side effects. With the development of medical immunology and molecular biology, immunotherapy aimed at enhancing the autoimmune system against tumors plays an increasingly important role in the comprehensive treatment of liver cancer. At present, the primary cancer immunotherapy strategies include tumor vaccines, blocking immune checkpoints, and adoptive cellular therapy.
It has been proven that BORIS sf6-targeted immunotherapy can recognize cervical CSCs and cancer-initiating cells (CICs), indicating that BORIS sf6 might be a promising candidate antigen for a tumor vaccine targeting cervical CSCs/CICs (13). Our previous studies have found that BORIS is highly expressed in HCC tissues and cells and is significantly correlated with invasion and recurrence, supporting the oncogenic properties of BORIS in human HCC (15,16). The expression of BORIS is mainly epigenetically regulated. It has been shown that the demethylation of the promoter leads to upregulated expression of BORIS in HCC (22). However, through epigenetic modification of the histone methylation status, rather than that of promoter CpG methylation, BORIS up-regulates OCT4 to promote CSC-like properties of HCC (19). Although high expression and tumorigenic activity of BORIS in HCC tissues and cells have been confirmed, there is no literature reports the use of BORIS as a new target for immunotherapy of HCC. So it’s urgent to explore the potential feasibility of tumor vaccines specifically activate cytotoxic lymphocytes (CTLs) to kill hepatoma cells by targeting BORIS, which may promote the immunotherapy of liver cancer. Among the sfs of BORIS, we were most curious about the immunogenicity of BORIS sf2 in liver cancer cells and its potential as a candidate polypeptide for a hepatocarcinoma vaccine. Animal models can provide an experimental platform for exploring the antitumor effects of tumor vaccines. Therefore, our study constructed a valuable murine tumor model to facilitate relevant animal investigations and better understand the therapeutic efficacy and limitations of a HCC vaccine. We expect that the model will prove to be an appropriate animal model for initiating the research on immunotherapy for liver cancer using BORIS sf2 as a target antigen.
This study is divided into three parts. Firstly, our preliminary study established a lentivirus-mediated stable cell line overexpressing BORIS sf2 using a lentiviral recombinant vector pCS-CG C2/A4. The plasmid was engineered to express a fusion protein containing the polypeptide encoded by BORIS sf2 and enhanced green fluorescent protein (EGFP). However, we could not screen the BORIS sf2-expressing cells due to the weak fluorescence emitted by the EGFP. We then performed relatively in-depth studies on the probable causes of this phenomenon via a gene integration analysis, an expression analysis of EGFP and BORIS sf2, and a spatial conformation prediction of EGFP in the fusion protein with BORIS sf2. Finally, we decided to obtain stable cell lines by screening puromycin resistance instead of fluorescence, so the lentiviral expression vector pCS-CG was replaced with pLVX-EF1α-IRES-Puro. Eventually, we achieved a stable expression of BORIS sf2/C68 in murine hepatoma cells. The Hepa1c1c7 cells expressing BORIS sf2/C68 (5×106/mouse) were subcutaneously injected into 6-week-old mice to induce the formation of subcutaneous tumors, and the tumor growth was monitored regularly. To summarize, this study provided a reliable experimental model for exploring the feasibility of a HCC vaccine targeting BORIS sf2 that could be specifically applied in the immunotherapy of liver cancer. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-6336/rc).
Methods
Animals and cells
The 293LTV cell line, human non-small cell lung cancer cell line A549, and the murine liver cancer cell lines Hepa1-6 and Hepa1c1c7 were purchased from Nanjing Kebai Biotechnology Co. Ltd. (Nanjing, China). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, USA) containing 10% fetal bovine serum (FBS) (Gibco, USA) and 100 units/mL of penicillin and 100 µg/mL of streptomycin (Thermo Scientific HyClone, China) in a humidified 37 ℃ incubator with 5% CO2. The C57BL/6 mice, aged 6 weeks and weighing 18–22 g, were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). The air in the animal breeding room was allowed to circulate, the temperature was kept around 25°C, and the humidity was maintained between 50% and 70%. Animal experiments were performed in Sichuan Xiapesen Medical Technology Co., Ltd. (No. SYXK(chuan)2017-203). All animal care and experiments were performed according to the institutional guidelines approved by the Animal Care and Use Committee of Sichuan Xiapesen Medical Technology Co., Ltd. A protocol was prepared before the study without registration.
Establishment of stable cell lines
The overexpressed lentiviral recombinant plasmid pCS-CG sf2 was constructed by Zhongjian Liu (a former Ph.D. student). Using the recombinant plasmid pCS-CG sf2 as a template, the cDNA sequences of BORIS sf2 and BORIS C68 were amplified by PCR. After identifying the target sequences by agarose gel electrophoresis, the two PCR amplification products were cloned into the pLVX-EF1α-IRES-Puro vector. The production of lentiviral particles using the 293LTV cell line and the subsequent infection of Hepa1-6, A549, and Hepa1c1c7 cells were performed according to the instruction manual. Positive cells overexpressing BORIS sf2 and BORIS C68 constructed with Hepa1c1c7 cells were selected with puromycin (Clontech, Mountain View, CA, USA) for 14 days.
Autophagy analysis
3-methyladenine (3-MA) were purchased from Shanghai McClaren Biochemical Technology Co., Ltd (Shanghai, China). When the viral supernatant was collected to infect Hepa1-6 cells and A549 cells, 3-MA was added to a final concentration of 5 mM or 10 mM, respectively, and the cells were subsequently allowed to grow in cell culture medium containing 3-MA with unchanged concentration to block autophagic proteolysis. The cells of the control groups were infected with the same virus supernatant, but they were not treated with 3-MA.
PCR analysis and RT-PCR analysis
Genomic DNA was extracted from the cells infected by Lenti-pCS-CG and Lenti-pCS-CG C2/A4 by the sodium dodecyl sulfate (SDS)-proteinase K-phenol-chloroform method (Tiangen Biochemical Technology Co. Ltd., Beijing, China). Then the genomic DNA was used as templates for the PCR amplification of EGFP and BORIS sf2. Total RNA was extracted using the RaPure Total RNA Micro Kit (Magen, Guangzhou, China), and cDNA was synthesized using the PrimerScriptTM RT Reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa, Dalian, China). The reverse transcriptase-polymerase chain reaction (RT-PCR) was performed with 2×PCR MasterMix (Invitrogen, Carlsbad, CA, USA). RT-PCR was performed in triplicate. The relative levels of mRNA were normalized to those of GAPDH.
Western blotting
The cultured cells were lysed in RIPA lysis buffer (Beyotime, Shanghai, China) supplemented with a cocktail protease inhibitor (Roche, Basel, Switzerland). The proteins were separated by SDS-PAGE and were transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). The membranes were blocked in a 5% nonfat milk solution for 2 h at 37 ℃ and were incubated with a primary antibody (Zen BioScience, Chengdu, China) at 4 ℃ overnight, followed by incubation with HRP-conjugated secondary antibodies (Beyotime, Shanghai, China) for 1 h. The signals were visualized using the BeyoECL Plus kit (Beyotime, Shanghai, China). β-actin was used as an internal control.
Tumor induction and growth experiments
To detect the in vivo tumorigenicity of the two stable cell lines, BORIS sf2-overexpressing Hepa1c1c7 cells [5×106 in 100 µL of phosphate-buffered saline (PBS)] and BORIS C68-overexpressing Hepa1c1c7 cells (5×106 in 100 µL of PBS) were harvested with trypsin, washed three times with PBS, and injected subcutaneously into the flanks of C57BL/6 mice (n=12 per group). The tumor size was monitored regularly for 41 days, and the tumor volume was determined according to the formula: tumor volume = ½ (length × width2). The data were used to plot a graph of the tumor growth curve.
Statistical analysis
All statistics were calculated using Graphpad Prism version 8 (GraphPad Software, San Diego, CA, USA). Statistical significance was determined using a one-way ANOVA to test for differences between multiple groups. The F test was used to compare variances of samples. Graphs show mean ± SD unless otherwise indicated. For all statistical data, P<0.05 was considered statistically significant.
Results
A failed establishment of stable cell lines expressing BORIS sf2 with the pCS-CG vector
The lentiviral packaging cell line 293LTV is a transformed cell line derived from the human primary embryonic kidney cell line 293 and is frequently used to produce lentiviral particles (23,24). To achieve the expression of fluorescent BORIS sf2-EGFP fusion proteins, we cloned the cDNA containing the BORIS sf2 gene sequence into the NheI and AgeI sites of the plasmid pCS-CG (Figure 1A). The 293LTV cells were firstly co-transfected with the following mixture of plasmids: overexpression recombinant vector or control plasmid (pCS-CG C2/A4, a plasmid containing human BORIS C2/A4, and pCS-CG, carrying the EGFP as its fluorescent tag), and packaging plasmid pSPA×2 and lentivirus outer membrane plasmid pMD2.G. The viral supernatants designated Lenti-pCS-CG and Lenti-pCS-CG C2/A4 were collected to infect the Hepa1-6 cells. Since few studies have reported that the virus particle packaged with the lentiviral expression vector pCS-CG can successfully infect murine tumor cells, we used the human non-small cell lung cancer cell line A549 as a species control when infecting murine hepatoma cells Hepa1-6 with the harvested virus supernatants. Fluorescence microscopy revealed that the packaging efficiencies of the 293LTV cells for Lenti-pCS-CG and Lenti-pCS-CG C2/A4 were equivalent (Figure 1B). Interestingly, the fluorescence intensity of the cells infected by Lenti-pCS-CG was strong (Figure 1C,1D), while the cells infected by Lenti-pCS-CG C2/A4 demonstrated weaker fluorescence, and the fluorescence decayed to an intensity that was undetectable by fluorescence microscopy after several cell passages (Figure 1C,1D), making it impossible to screen the target cells with apparent and enduring fluorescence by flow cytometry. Since this contrast was present in both Hepa1-6 and A549 cells, the fluorescence inefficiency in the mouse-derived cells infected by Lenti-pCS-CG C2/A4 could not have been caused by the difference in species.

The weak fluorescence was associated with a reduced EGFP protein expression in the fusion protein
We conducted a series of experimental analyses to find a reasonable explanation for the faint fluorescence emission of the EGFP encoded by the engineered recombinant plasmid pCS-CG C2/A4. Lentivirus is an RNA virus containing reverse transcriptase. In the process of lentiviral infection of target cells, cDNA is firstly synthesized by reverse transcription using viral RNA as a template. Under the integrase catalysis, the viral DNA genome integrates into the chromosome of host cells. The viral RNA, structural protein, and non-structural protein, which finally assemble to generate complete viral particles, are formed by transcription and translation. Therefore, we can evaluate the infection efficiency of the lentivirus by detecting the gene integration. Meanwhile, the mRNA expression of BORIS sf2 and EGFP in the lentiviral genome can be analyzed by RT-PCR. These two experiments were designed to help us determine whether the weak infection of lentivirus in the target cells or the inefficient transcription of the EGFP gene contributed to the nearly invisible fluorescence of the cells infected by Lenti-pCS-CG C2/A4. The experimental results did not provide support for either of these two speculations because the lentiviral infection rates and the expression of EGFP at the mRNA level did not demonstrate significant differences between the cells infected by Lenti-pCS-CG and Lenti-pCS-CG C2/A4 (Figure 2A,2B). In addition, it was clear that, according to the gene integration analysis, the human tumor cell line A549 was not more susceptible to the lentivirus infection compared to the murine cell line Hepa1-6. Based on the invalidation of the above assumptions, we then attempted to verify whether the EGFP in the fusion protein maintained a continuous and stable expression at the protein level by using a western blot assay. The result revealed that the Hepa1-6 cells infected by Lenti-pCS-CG C2/A4 expressed almost no EGFP compared with cells infected by Lenti-pCS-CG (Figure 2C). Therefore, we could justifiably conclude that pCS-CG cannot be used as a fusion expression vector for screening BORIS sf2 expressing cells with a fluorescent EGFP tag. Although decreased EGFP protein expression in the fusion protein appears to be a reasonable explanation for the weakened fluorescence, we have not yet determined what mechanism seriously interfered with the EGFP protein expression. Another crucial factor for normal EGFP fluorescence emission is the maintenance of its proper spatial structure, so we used the I-TASSER software which can serve for protein 3D structure prediction to simulate the three-dimensional structure of this conformationally unknown chimeric protein (25). We compared the predicted structure with the crystallographic structure of EGFP (acquired from RCSB PDB:2Y0G). It was clear that these two proteins both possessed considerably compact tertiary structures which resembled a barrel (26,27). The topological similarity of protein structure pairs is commonly evaluated using the parameter root mean square deviation (RMSD), and the high-resolution models with RMSD in 1–2 Å are typically generated by comparative modeling using close homologous templates (25). The RMSD of the steric conformation compared in our study was 1.16 Å, which means the fusion of the N-terminus of EGFP to the C-terminus of BORIS sf2 produced a protein that underwent correct folding which retained the original conformation of EGFP. Consistent with the implication of this parameter, we could see that the EGFP encoded by the unmodified vector pCS-CG and by the artificial recombinant plasmid pCS-CG C2/A4 exhibited negligible differences in spatial conformations by merging their 3D shapes (Figure 2D). Consequently, the presumption that weak autofluorescence was caused by a change in EGFP spatial conformation could not be upheld.
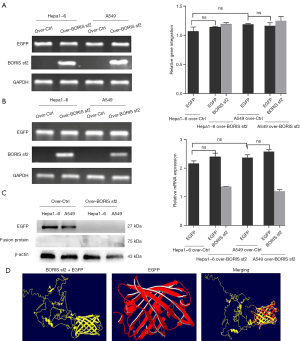
Exogenous overexpression of BORIS sf2 leads to autophagy and unfolded protein response (UPR) of cells
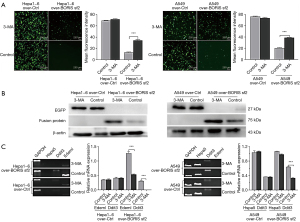
Decreased protein expression is mainly caused by lessened protein synthesis or increased protein degradation. Cycloheximide, a translational inhibitor, was originally planned in our study to measure degradation kinetics for BORIS sf2-EGFP fusion protein. However, the Hepa1-6 and A549 cells infected by Lenti-pCS-CG C2/A4 constantly showed faint fluorescence without a peak of fluorescence intensity, so the dynamic degradation process of the fusion protein couldn’t be monitored. The human BORIS sf2 is expressed in Hepa1-6 and A549 cells as an exogenous protein, which may be cleared as an unfavorable molecule by lysosome through the autophagic pathway (28). 3-MA is a classical autophagy inhibitor that mainly plays an inhibitory role in the formation and development of autophagosomes (29). To investigate whether the cells underwent autophagy in response to the overexpression of the exogenous molecule BORIS sf2, virus supernatant containing 3-MA was used to infect the targeted cells, while cells of the control groups were not treated with 3-MA. Interestingly, Hepa1-6 and A549 cells infected by Lenti-pCS-CG C2/A4 and treated with 3-MA emitted more pronounced green fluorescence than the control cells without 3-MA treatment (Figure 3A). We then quantitatively analyzed the mean fluorescence intensity of the two groups of cells using the software Image J. The results demonstrated that there are statistically significant differences in the mean fluorescence intensity presented by cells of these two groups (Figure 3A). However, the cell lines Hepa1-6 and A549 infected by Lenti-pCS-CG and treated with 3-MA showed almost indistinguishable fluorescence intensity compared to the control cells (Figure 3A). We performed the Western Blot assay to determine whether the cells infected by Lenti-pCS-CG C2/A4 and treated with 3-MA expressed increased BORIS sf2-EGFP fusion protein. The results suggested that enhanced fluorescence was associated with increased expression of the fusion protein (Figure 3B). Endoplasmic reticulum (ER) is one of the important organelles to ensure the normal physiological functions of cells. When the uptake and release of Ca2+ by ER is disturbed or the processing and transportation of proteins are impaired, it will lead to endoplasmic reticulum stress (ERS). The translation of unfolded or misfolded proteins due to accumulation in the ER lumen can lead to UPR. Typically, what is commonly referred to as ERS is mainly caused by UPR (30). There is a tight connection between UPR and autophagy, both of which can play an important protective role in tumor cells in a stressful environment (31). Many important molecules in the UPR can promote the occurrence of autophagy, such as ATF4, CHOP and TFEB (32,33). Thus we’re curious about whether the UPR also occurred in the cells overexpressing BORIS sf2. The UPR gene signatures, including Hspa5, Ddit3 and Edem1 (34), were examined by RT-PCR analysis. Results indicated that there was higher expression of Edem1 and Ddit3 in Hepa1-6 cells overexpressing BORIS sf2, but the expression of these two genes was decreased by treatment of 3-MA (Figure 3C). However, only Ddit3 was more prominently expressed in A549 overexpressing BORIS sf2. Similarly, 3-MA depressed the expression of Ddit3 (Figure 3C). In conclusion, the tumor cells Hepa1-6 and A549 initiate UPR and autophagic response to degrade the exogenous BORIS sf2/EGFP fusion protein, which has largely brought about the faint green fluorescence in the cells infected by Lenti-pCS-CG C2/A4.
Establishment of hepatoma cell lines expressing BORIS sf2/C68 with the lentiviral expression vector pLVX-EF1α-IRES-Puro
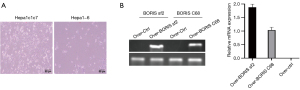
The screening of our target cells was an undeniable challenge posed by the weak fluorescence, so we replaced the lentiviral expression vector pCS-CG with pLVX-EF1α-IRES-Puro, which carries the puromycin resistance gene as its screening marker, rather than EGFP. Considering that BORIS sf2 contains a specific C-terminal domain (C68), a vaccine explicitly targeting the CT antigen BORIS sf2 could also be achieved by targeting these 68 amino acids. Next, we cloned the cDNA containing the BORIS sf2/C68 gene sequences into the EcoRI and BamHI sites of the vector pLVX-EF1α-IRES-Puro to construct the corresponding lentiviral recombinant plasmids. Then, the same procedures for lentiviral packaging and infection of target cells were performed as mentioned before. Since lentiviral infection created an adverse effect on the growth of Hepa1-6 cells, we compared the tolerance of the two hepatoma cell lines, Hepa1-6 and Hepa1c1c7, to lentiviral infection. Finally, we selected the Hepa1c1c7 due to its more robust survivability after virus infection (Figure 4A). The puromycin-resistant cells were finally obtained after we allowed the lentivirus-infected Hepa1c1c7 cells to grow in a complete medium containing puromycin at a concentration of 4 µg/mL for 14 days and the surviving cells were subsequently expanded for culture. RT-PCR was carried out to ascertain the expression of BORIS sf2/C68 at the mRNA level (Figure 4B). The results of this study demonstrate that we have successfully established a murine hepatoma cell line overexpressing a human-derived antigen, the BORIS sf2/C68. We envisage that when immunization with a HCC vaccine is administered to mice bearing BORIS sf2/C68-positive tumors, these inoculated hepatoma cells cannot avoid immune responses directed at BORIS sf2/C68.
Creation of a tumor model which can offer human-derived molecular targets
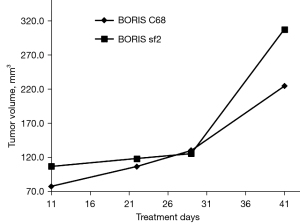
To detect the tumorigenicity of BORIS sf2/C68-overexpressing liver cancer cells and establish a tumor-bearing mouse model, we subsequently performed a tumor formation assay with immunocompetent C57BL/6 mice. The mice were divided into two groups, and tumor growth kinetics were determined by injecting BORIS sf2- and C68-expressing Hepa1c1c7 cells subcutaneously into the mice, respectively. The tumor volume was measured by caliper measurements in two perpendicular dimensions and the temperature and mental state of mice were monitored regularly. In both groups, almost 100% of mice injected with the Hepa1c1c7 cells at a dose of 5×106 cells/mouse displayed tumor formation and progressive tumor growth (Figure 5). We made the tumor volume generated in the experimental animals did not surpass 1,200 mm3. Furthermore, weak growth was extremely rare in these animals. In fact, the induced BORIS sf2/C68-expressing tumors closely mirrored partial human HCC. Our studies, therefore, suggest that we have constructed a murine cancer model that may serve as a highly suitable model for studying immune reactions against BORIS sf2-associated HCC.
Discussion
As the sole known paralog of CTCF, BORIS is expressed as 23 mRNA splice variants which have the potential to encode 17 different isoproteins (20). Among the BORIS subfamilies divided according to unique 3’ terminal sequences, the BORIS sf2 has only three ZF domains and a distinct C68 domain. Constructing animal models is essential for the development of cancer vaccines. To date, some well-established animal models have been used to study cancer prevention or treatment strategies. A Johns Hopkins University research team successfully constructed a murine cervical cancer model with TC-1 cells expressing HPV16 E6 and E7 genes for therapeutic tumor vaccine research, and Smahel et al. developed a model with secondary C57BL/6 murine kidney cells for studying the immunotherapy of cancers associated with human papillomaviruses (HPVs) (28,29). Similarly, the animal tumor model (MC38-CEA) constructed from the murine colon carcinoma cell line MC38 expressing human carcinoembryonic antigen (CEA) might be particularly useful for optimizing various forms of immunotherapy, using CEA as a target antigen (30-32). The primary limitation in human HCC immunotherapy studies is the lack of applicable preclinical models, which makes clinical research challenging to implement. To date, of the therapeutic options for liver cancer, an immunotherapeutic strategy directed at the antigen BORIS sf2 has not been described. Therefore, we conducted this research to establish an animal model to test whether cancer immunotherapy using BORIS sf2/C68 is a promising approach for treating chemotherapy- and radiotherapy-resistant liver cancers.
The expression of EGFP, a variation of GFP, generates striking green fluorescence, facilitating its extensive use as an excellent reporter of gene expression and protein localization (33,34). In our study, we successfully inserted BORIS sf2 into the plasmid pCS-CG, but weak EGFP protein expression resulted in nearly invisible fluorescence in the cells infected by Lenti-pCS-CG C2/A4. A previous study reported cleavage of the fusion protein containing EGFP as a fusion partner and suggested this was probably caused by the presence of substances that degraded the fusion protein (35). Toca-Herrera et al. also pointed out that the intensity of EGFP fluorescence emission was related to temperature, pH conditions, and guanidine hydrochloride concentrations (36). It has also been reported that, compared to separate proteins, artificial protein fusion can significantly impact the total conformational stability, which negatively affects the fluorescence emission of EGFP (37,38). The majority of misfolded proteins generated in ER are dislocated across the membrane for degradation by proteasomes to prevent toxic accumulation of aberrant gene products. But lysosomal clearance of adverse proteins will happen with a higher probability when the cells are exogenously stimulated (39). In our study, when Hepa1-6 and A549 cells were infected by Lenti-pCS-CG C2/A4, the autophagic response triggered by the expression of BORIS sf2 led to degradation of the fusion protein.
In this study, although the construction of a stable cell line was not successful initially, we empirically demonstrated a new and experimentally relevant insight that reminds us that screening out desired cells by forming a fusion protein with commonly used fluorescent tags is not always achievable. Eventually, we achieved a stabilized expression of BORIS sf2/C68 in oncogenic Hepa1c1c7 cells with a lentivirus-mediated approach using another lentiviral vector, pLVX-EF1α-IRES-Puro, which carries the puromycin-resistance gene. The expression of transformed genes was detected at the mRNA level by RT-PCR. Finally, the two stable cell lines expressing BORIS sf2 and C68 were implanted subcutaneously into the right flank of mice to induce subcutaneous tumors. During the tumor formation experiment, the challenge cells exhibited a strong carcinogenic potential, and tumor sizes were recorded regularly. According to the tumor growth curves, the tumor volumes generated by BORIS sf2-positive cells were generally larger, which suggested that the cells were intrinsically more tumorigenic than the BORIS C68-expressing cells. One possible explanation is that BORIS sf2 was more carcinogenic due to the oncogenesis of the DNA-binding ZF domain (17,18,40).
In conclusion, the animal model we constructed can be used to investigate whether BORIS sf2 is immunogenic in mice, which is critical in determining whether antigen BORIS sf2 is a promising candidate for a HCC vaccine. Of course, several deficiencies exist in our study. Firstly, although we established that the weak fluorescence was related to decreased EGFP protein expression, the critical factors leading to this phenomenon remain unknown. Additionally, we were unable to perform the western blot assay to confirm the expression of BORIS sf2 and C68 in the Hepa1c1c7 cells due to the lack of antibody reagent. Therefore, our laboratory team is presently trying to produce the antibody specific to BORIS sf2, and we expect to provide more robust evidence for the expression of this CT antigen through immunoblotting in a future study.
Acknowledgments
Funding: This research was supported by a grant from the Technology Innovation R & D Project Foundation of Chengdu (No. 2019YF-05-00369-SN).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-6336/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-6336/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-6336/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were performed in Sichuan Xiapesen Medical Technology Co., Ltd. [No. SYXK(chuan)2017-203]. All animal care and experiments were performed according to the institutional guidelines approved by the Animal Care and Use Committee of Sichuan Xiapesen Medical Technology Co., Ltd.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Loukinov DI, Pugacheva E, Vatolin S, et al. BORIS, a novel male germ-line-specific protein associated with epigenetic reprogramming events, shares the same 11-zinc-finger domain with CTCF, the insulator protein involved in reading imprinting marks in the soma. Proc Natl Acad Sci U S A 2002;99:6806-11. [Crossref] [PubMed]
- Marshall AD, Bailey CG, Rasko JE. CTCF and BORIS in genome regulation and cancer. Curr Opin Genet Dev 2014;24:8-15. [Crossref] [PubMed]
- Klenova EM, Morse HC 3rd, Ohlsson R, et al. The novel BORIS + CTCF gene family is uniquely involved in the epigenetics of normal biology and cancer. Semin Cancer Biol 2002;12:399-414. [Crossref] [PubMed]
- Martin-Kleiner I. BORIS in human cancers -- a review. Eur J Cancer 2012;48:929-35. [Crossref] [PubMed]
- de Necochea-Campion R, Ghochikyan A, Josephs SF, et al. Expression of the epigenetic factor BORIS (CTCFL) in the human genome. J Transl Med 2011;9:213. [Crossref] [PubMed]
- Freitas M, Malheiros S, Stávale JN, et al. Expression of cancer/testis antigens is correlated with improved survival in glioblastoma. Oncotarget 2013;4:636-46. [Crossref] [PubMed]
- D'Arcy V, Abdullaev ZK, Pore N, et al. The potential of BORIS detected in the leukocytes of breast cancer patients as an early marker of tumorigenesis. Clin Cancer Res 2006;12:5978-86. [Crossref] [PubMed]
- D'Arcy V, Pore N, Docquier F, et al. BORIS, a paralogue of the transcription factor, CTCF, is aberrantly expressed in breast tumours. Br J Cancer 2008;98:571-9. [Crossref] [PubMed]
- Okabayashi K, Fujita T, Miyazaki J, et al. Cancer-testis antigen BORIS is a novel prognostic marker for patients with esophageal cancer. Cancer Sci 2012;103:1617-24. [Crossref] [PubMed]
- Risinger JI, Chandramouli GV, Maxwell GL, et al. Global expression analysis of cancer/testis genes in uterine cancers reveals a high incidence of BORIS expression. Clin Cancer Res 2007;13:1713-9. [Crossref] [PubMed]
- Hoivik EA, Kusonmano K, Halle MK, et al. Hypomethylation of the CTCFL/BORIS promoter and aberrant expression during endometrial cancer progression suggests a role as an Epi-driver gene. Oncotarget 2014;5:1052-61. [Crossref] [PubMed]
- Woloszynska-Read A, James SR, Link PA, et al. DNA methylation-dependent regulation of BORIS/CTCFL expression in ovarian cancer. Cancer Immun 2007;7:21. [PubMed]
- Asano T, Hirohashi Y, Torigoe T, et al. Brother of the regulator of the imprinted site (BORIS) variant subfamily 6 is involved in cervical cancer stemness and can be a target of immunotherapy. Oncotarget 2016;7:11223-37. [Crossref] [PubMed]
- Garikapati KR, Patel N, Makani VKK, et al. Down-regulation of BORIS/CTCFL efficiently regulates cancer stemness and metastasis in MYCN amplified neuroblastoma cell line by modulating Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun 2017;484:93-9. [Crossref] [PubMed]
- Chen K, Huang W, Huang B, et al. BORIS, brother of the regulator of imprinted sites, is aberrantly expressed in hepatocellular carcinoma. Genet Test Mol Biomarkers 2013;17:160-5. [Crossref] [PubMed]
- Liu Q, Chen K, Liu Z, et al. BORIS up-regulates OCT4 via histone methylation to promote cancer stem cell-like properties in human liver cancer cells. Cancer Lett 2017;403:165-74. [Crossref] [PubMed]
- Mkrtichyan M, Ghochikyan A, Loukinov D, et al. DNA, but not protein vaccine based on mutated BORIS antigen significantly inhibits tumor growth and prolongs the survival of mice. Gene Ther 2008;15:61-4. [Crossref] [PubMed]
- Loukinov D, Ghochikyan A, Mkrtichyan M, et al. Antitumor efficacy of DNA vaccination to the epigenetically acting tumor promoting transcription factor BORIS and CD80 molecular adjuvant. J Cell Biochem 2006;98:1037-43. [Crossref] [PubMed]
- Mkrtichyan M, Ghochikyan A, Davtyan H, et al. Cancer-testis antigen, BORIS based vaccine delivered by dendritic cells is extremely effective against a very aggressive and highly metastatic mouse mammary carcinoma. Cell Immunol 2011;270:188-97. [Crossref] [PubMed]
- Pugacheva EM, Suzuki T, Pack SD, et al. The structural complexity of the human BORIS gene in gametogenesis and cancer. PLoS One 2010;5:e13872. [Crossref] [PubMed]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17. [Crossref] [PubMed]
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27. [Crossref] [PubMed]
- Pourfath MR, Behzad-Behbahani A, Hashemi SS, et al. Monitoring wound healing of burn in rat model using human Wharton's jelly mesenchymal stem cells containing cGFP integrated by lentiviral vectors. Iran J Basic Med Sci 2018;21:70-6. [PubMed]
- Bhattacharya S, Saini M, Bisht D, et al. Lentiviral-mediated delivery of classical swine fever virus Erns gene into porcine kidney-15 cells for production of recombinant ELISA diagnostic antigen. Mol Biol Rep 2019;46:3865-76. [Crossref] [PubMed]
- Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 2010;5:725-38. [Crossref] [PubMed]
- Ormö M, Cubitt AB, Kallio K, et al. Crystal structure of the Aequorea victoria green fluorescent protein. Science 1996;273:1392-5. [Crossref] [PubMed]
- Yang F, Moss LG, Phillips GN Jr. The molecular structure of green fluorescent protein. Nat Biotechnol 1996;14:1246-51. [Crossref] [PubMed]
- Lin KY, Guarnieri FG, Staveley-O'Carroll KF, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res 1996;56:21-6. [PubMed]
- Smahel M, Sobotková E, Bubeník J, et al. Metastatic MHC class I-negative mouse cells derived by transformation with human papillomavirus type 16. Br J Cancer 2001;84:374-80. [Crossref] [PubMed]
- Eades-Perner AM, van der Putten H, Hirth A, et al. Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expression pattern. Cancer Res 1994;54:4169-76. [PubMed]
- Clarke P, Mann J, Simpson JF, et al. Mice transgenic for human carcinoembryonic antigen as a model for immunotherapy. Cancer Res 1998;58:1469-77. [PubMed]
- Muders M, Ghoreschi K, Suckfuell M, et al. Studies on the immunogenicity of hCEA in a transgenic mouse model. Int J Colorectal Dis 2003;18:153-9. [Crossref] [PubMed]
- Chalfie M, Tu Y, Euskirchen G, et al. Green fluorescent protein as a marker for gene expression. Science 1994;263:802-5. [Crossref] [PubMed]
- Yang TT, Cheng L, Kain SR. Optimized codon usage and chromophore mutations provide enhanced sensitivity with the green fluorescent protein. Nucleic Acids Res 1996;24:4592-3. [Crossref] [PubMed]
- Raschmanová H, Paulová L, Branská B, et al. Production and cleavage of a fusion protein of porcine trypsinogen and enhanced green fluorescent protein (EGFP) in Pichia pastoris. Folia Microbiol (Praha) 2018;63:773-87. [Crossref] [PubMed]
- Toca-Herrera JL, Küpcü S, Diederichs V, et al. Fluorescence emission properties of S-Layer enhanced green fluorescent fusion protein as a function of temperature, pH conditions, and guanidine hydrochloride concentration. Biomacromolecules 2006;7:3298-301. [Crossref] [PubMed]
- Yantsevich AV, Gilep AA, Usanov SA. Conformational stability of cytochrome b5, enhanced green fluorescent protein, and their fusion protein Hmwb5-EGFP. Biochemistry (Mosc) 2009;74:518-27. [Crossref] [PubMed]
- Li X, Zhao X, Fang Y, et al. Generation of destabilized green fluorescent protein as a transcription reporter. J Biol Chem 1998;273:34970-5. [Crossref] [PubMed]
- Fregno I, Molinari M. Proteasomal and lysosomal clearance of faulty secretory proteins: ER-associated degradation (ERAD) and ER-to-lysosome-associated degradation (ERLAD) pathways. Crit Rev Biochem Mol Biol 2019;54:153-63. [Crossref] [PubMed]
- Ghochikyan A, Mkrtichyan M, Loukinov D, et al. Elicitation of T cell responses to histologically unrelated tumors by immunization with the novel cancer-testis antigen, brother of the regulator of imprinted sites. J Immunol 2007;178:566-73. [Crossref] [PubMed]


