Increased cerebellar activation after repetitive transcranial magnetic stimulation over the primary motor cortex in patients with multiple system atrophy
Introduction
Multiple system atrophy (MSA) is a rare, distinct and devastating neurodegenerative disease, characterized by a combination of symptoms that affect both the autonomic nervous system and motor system (1,2). The hallmark of its neuropathology is glial cytoplasmic inclusions composed of filamentous α-synuclein proteins in the striato-nigral and olivo-ponto-cerebellar structures (3). Some of the motor symptoms, such as bradykinesia, rigidity, gait instability, and tremor, are similar to those of Parkinson’s disease (PD) (4). Patients with predominant parkinsonian features are defined as multiple system atrophy with Parkinsonism (MSA-P) (1). The pathophysiology of motor impairment in MSA remains largely unclear (5). Besides, pharmacological treatment has limited effects on the improvement of motor symptoms of MSA patients (1). Typical antiparkinsonian therapy for PD, such as dopamine replacement therapy, doesn’t work for MSA patients (1).
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive neuromodulation technique that has been examined as a possible treatment for neurodegenerative diseases (6,7). It delivers repeated magnetic pulses through a stimulation coil placed over the scalp to generate a relatively focal electromagnetic field capable of triggering action potentials in neurons (8). rTMS can modulate cortical excitability, so that it has been increasingly utilized for various neurological (9) and psychiatric conditions (10,11). Evidence has shown that rTMS is potentially helpful in alleviating motor symptoms for patients with PD (12). And it was observed from a meta-analysis that the rTMS effects were stronger and significant when high-frequency (≥5 Hz) rTMS was targeted at the primary motor cortex (M1) (6). Given the limited efficacy of pharmacological treatment in improving motor symptoms of MSA (1), there is a clinical need to determine whether and how rTMS could benefit this population.
The first aim of this study was to examine the effect of high-frequency rTMS over the left M1 on motor symptoms in MSA patients. The second was to use a finger-tapping task to assess the rTMS-induced functional modulation in task related brain activations.
Materials and methods
Subjects
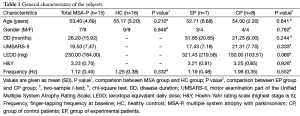
Fifteen right-handed MSA patients with were prospectively enrolled in this study. All patients fulfilled the diagnosis of probable MSA with predominantly parkinsonian according to the established consensus criteria (1). Exclusion criteria were significant medical or psychiatric illnesses, history of epilepsy or seizures, pregnancy, or mental diseases. All of these patients did not respond well to the levodopa treatment. Seven patients were randomly assigned to the experimental patient group (EP group) and 8 patients were assigned to the control patient group (CP group). Patients were not aware of the specifics of the experimental design. In addition, 18 healthy controls (HC group) were prospectively enrolled in this study. Healthy subjects had normal neurological examination and none had a history of hypertension, diabetes mellitus, cerebral vascular diseases, or other neurological disorders. Subjects with white matter lesions were excluded. Characteristics of the study population are summarized in Table 1. The study was approved by the institutional review board of Peking Union Medical College Hospital, and all subjects gave written informed consent.
Full table
Procedures
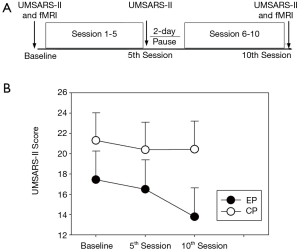
The overall experimental procedure consisted of two functional magnetic resonance imaging (fMRI) sessions, before and after a 10-session rTMS treatment. Subjects from the HC group did not receive any rTMS treatment and they underwent fMRI examination only once. A schematic of the various stages and their timing is shown in Figure 1, with their details described below.
Transcranial magnetic stimulation
A 65-mm figure eight-shaped coil (MCF-B65) and a MagPro Compact stimulator (Dantec Company, Copenhagen, Denmark) were used in the rTMS sessions. We first determined the optimal scalp location of the left M1 for rTMS treatment. The resting motor threshold (RMT) of the right abductor digiti minimi (ADM) muscle was measured for each patient. RMT was defined as the lowest intensity capable of eliciting at least five motor-evoked potentials of 50 µV peak-to-peak amplitude in ten consecutive stimulations when single-pulse TMS was delivered to the left M1. Electromyography recordings from the right ADM were acquired with surface electrodes using a Viking IV electromyography machine (Nicolet Biomedical, Madison, WI, USA). Bandpass filters were set at 20–2,000 Hz.
Patients of EP group received real rTMS treatment. The rTMS protocol was based on published studies, which demonstrated that 5 Hz rTMS therapy improved motor symptoms in patients with PD (13-15). Ten sessions of rTMS were delivered over 2 weeks, one session per day for 5 consecutive days per week. Each session consisted of 10 trains of 100 pulses at 5 Hz with an inter-train interval of 40 seconds. The intensity was set to 110% RMT. The figure-eight shaped 65 mm coil, connected to Medtronic MagPro stimulator (Denmark), was positioned over the left M1 corresponding to the hotspot of right ADM, and fixed to a coil holder.
Patients in the sham rTMS group received the same rTMS procedure targeting the left M1, except that the coil was positioned with its back (inactive) surface touching the scalp (15). In our pilot study, we measured electric field power induced by the figure eight-shaped coil with the front and the back surfaces using a testing coil (MagProbe) and the electromyography machine. The data showed a reduced power of electric field by 92% when the coil was placed with its back surface touching the scalp (1.0 mV) relative to the active stimulation (11.9 mV). A certified neurologist observed the procedure to ensure an optimal conduction for each patient and for safety monitoring. Each patient received rTMS intervention at the same time every morning (11:00 AM). Anti-parkinsonian medications around this time were not permitted to take as usual until at least 60 min after receiving the rTMS stimulation.
Clinical rating scale
The severity of parkinsonism was evaluated for all the patients with the motor score of the Unified Multiple System Atrophy Rating Scale (UMSARS-II) (16). The UMSARS has been validated to assess rates of progression and is sensitive to change over time (17). The UMSARS-II contains 14 questions with highest score representing more sever signs or symptoms. The UMSARS-II measure of the right-side limbs were obtained from patients at baseline and within one hour following the 5th and the 10th sessions of rTMS intervention.
Motor task
The task was a relatively complex self-paced sequential tapping movement performed with the right hand as used in other studies (18-20). Four task blocks were alternated with four subsequent rest blocks and each block lasted 30 s. During the task blocks, subjects were asked to tap each finger to the thumb in a specific order (i.e., index, middle, ring and little finger) and repeat this series of movements during the 30 s of data acquisition. No practice was performed before fMRI scanning except for simple introduction of the task to all the subjects. Subjects were instructed to perform the task at a frequency of 1 Hz approximately, meanwhile, they should make sure that each finger-to-thumb movement could be clearly seen and counted (21). During the scanning, subjects were restricted with their right upper arm close to the trunk so that they could not move any other part of the body except the right hand, and they were asked to ignore the scanning noise. Subjects started and ended their finger movements according to an acoustic signal in order to switch from task block to rest block and vice versa. During the rest blocks, subjects were instructed to remain still and keep their eyes closed. An operator was present in the magnetic resonance imaging (MRI) room throughout the session to ensure tasks being appropriately performed and record the number of taps to obtain the frequency (number/30).
fMRI scanning
All MRI exams were performed on a 3.0-T Signa Excite II VHi MR scanner (GE Healthcare, Milwaukee, Wisconsin, USA) equipped with a standard bird-cage head coil in Peking Union Medical College Hospital. Foam pads and ear plugs were used to reduce head motion and scan noise. Before the functional scan, 3-dimensional high-resolution T1-weighted gradient-echo images (TR =6.9 ms, TE =3.3 ms, T1 =400 ms, FA =15, FOV =240×240 mm2, matrix =256×256, slice number =164) were acquired for anatomical reference. Functional T2* weighted images were acquired with EPI sequence (TR =3,000 ms, TE =30 ms, FA =75º, FOV =192×192 mm2, voxel size =3.75×3.75×6 mm3, slice number =20). The subjects were instructed to keep their eyes open during fMRI data acquisition.
Statistical analysis
Group statistics were analyzed using SPSS for Windows. Age, disease duration, UMSARS-II score at baseline, levodopa dose and Hoehn-Yahr stage were assessed using two-sample t-tests to determine group differences. Gender was compared using chi-square test. Two-sample t-test was performed to compare finger-tapping frequency between MSA group and HC group. A 2×2 two-way mixed design analysis of variance (ANOVA) (factor 1: groups; factor 2: time) was performed to compare finger-tapping frequency between EP group and CP group. The overall UMSARS-II score of each patient was log transformed to improve the normality for statistical analyses. To examine whether active rTMS was more effective relative to the sham rTMS on changes in the UMSARS-II score, we performed a 2×3 two-way mixed design ANOVA with groups (EP vs. CP) as an independent factor and time (baseline vs. 5th session vs. 10th session) as a repeated factor. The Greenhouse-Geisser correction was used when necessary to correct for non-sphericity. A threshold of P<0.05 was used to determine statistical significance. Post-hoc tests were performed when the interaction effect was significant.
fMRI data analysis
Functional MRI data were preprocessed using SPM8 software (www.fil.ion.ucl.ac.uk/spm/). Volumes of each session were realigned to the first volume of each session for motor correction. Datasets with more than 2 mm maximum translation or 2 degree of maximum rotation along any axis were discarded. Then the functional images were spatially normalized to stereotaxic space of Talairach and Tournoux (22) and standardized with a Gaussian filter of 8 mm full-width at half-maximum to reduce noise. The final voxel size was 2×2×2 mm3.
A first-level analysis based on the general linear model (23) was performed for each subject individually. A high-pass filter (cutoff 128 s) was applied to remove low frequency temporal drifts in fMRI signal. The movement parameters obtained during realignment were also included in this model in addition to task condition. For each subject, one linear contrast of interest was calculated, corresponding to the effect of finger opposition movements minus that of the rest periods. Each of these linear contrasts was used for second-level random-effects analysis.
One-sample t-tests were performed to obtain task related activation for MSA and HC respectively. Two-sample t-test was performed for between-group comparisons of MSA and HC, and paired t-tests were performed for within-group comparisons between pre-rTMS and post-rTMS for both patient groups. Age, disease duration, dopaminergic medication and frequency of finger-tapping was controlled while performing between-groups comparison. Significance was considered when P<0.005 (uncorrected for multiple comparisons) and the spatial extents >10 voxels. All the activations were displayed by superimposing the T statistic maps on a standard high-resolution T1-weighted MRI brain template.
Parameter estimations of brain areas which showed significant changes after real rTMS treatment were extracted. Then correlation analyses were performed to investigate relationship between activation changes with UMSARS-II score changes.
Results
Clinical analysis
No significant side effect of rTMS treatment was reported by the patients. Before participation, all patients were informed orally and in a written form that they would be randomly assigned into either the EP group or CP group. At the end of the study, participants were interviewed regarding their expectation of benefits and group assignment. All of the patients thought that they had received real and active rTMS treatment. The age and gender was not significantly different between MSA group and HC group (Table 1). The UMSARS-II score at baseline, age, gender, disease duration, estimated levodopa dosage, and Hoehn-Yahr stage were not significantly different between EP group and CP group (Table 1).
Figure 1 illustrates the UMSARS-II score at different time points for MSA-P patients. An ANOVA of the UMSARS-II score yield a significant main effect of time [F (1.355, 17.609) =9.988, P=0.001] and a significant group × time interaction effect [F (2, 26) =6.320, P=0.006]. Post-hoc tests revealed EP group showed significant decreased UMSARS-II score after 10th session of active rTMS treatment relative to the score at baseline (P=0.001), and the sore after 5th session (P=0.000). For CP group, post-hoc tests showed no significant change of UMSARS-II score after rTMS treatment.
Task performance
All subjects were able to correctly execute the task, and none of them exhibited unwanted visible movements. Group average frequency was given as mean (SD) for each group (Table 1). Before rTMS treatment, MSA and HC showed no statistically significant difference in tapping frequency {t [31] =−0.986, P=0.332}. The tapping frequencies were statistically undistinguishable between the EP group and the CP group at baseline {t [13] =0.610, P=0.552}. An ANOVA of the frequency yielded no significant main effect of time [F (1, 14) =3.654, P=0.078] and no significant group × time interaction [F (1, 14) =0.040, P=0.845].
fMRI analysis
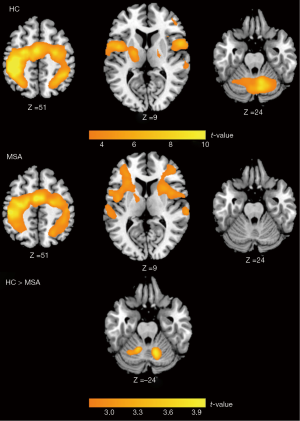
During the finger-tapping task, activation was predominantly observed in the motor areas, including the primary sensori-motor cortex, supplementary motor area (SMA), premotor cortex (PMC), inferior parietal cortex, inferior frontal cortex, and cerebellum in HC group. MSA patients showed significant activation over similar brain areas except for the cerebellum. Comparison between MSA and HC showed significant difference in the bilateral cerebellum (Figure 2).
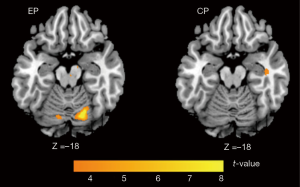
Within group comparison showed that increased activation was obtained in the bilateral cerebellum after rTMS treatment for the EP group. On the contrary, no increased activation was identified than the baseline for the CP group (Figure 3). No correlation was found between increase of cerebellum activation and decrease of UMSARS score for EP group.
Discussion
The present study investigated the effect of rTMS over the left M1 of patients with MSA. First, we identified that the rTMS treatment improved motor function for the real rTMS group, but not for the sham one. The mean score change in the UMSARS-II after 10 sessions of rTMS treatment was 3.64±2.58 for the EP group. Although the minimal clinically important difference (CID) for the UMSARS-II score has not been documented, the improvement in the UMSARS-II score observed in this study was greater that the minimal CID (2.3–2.7 points) for the Unified Parkinson’s Disease Rating Scale motor score (UPDRS-III, which has similar number of items and the same range of scores relative to the UMSARS-II) (24). Therefore, we believed that the improvement in UMSARS-II score might be clinically relevant.
Second, increased activation was observed in the bilateral cerebellum after rTMS treatment in the EP group. On the contrary, no increased activation was identified in the CP group. Cerebellum has long been recognized as an important site in the coordination of voluntary movement, gait posture and motor functions (25). Increasing anatomical, pathophysiological and functional neuroimaging evidences suggested that the cerebellum may contribute substantially to the function of basal ganglia. Recent literature showed that the cerebellum has a strong disynaptic projection to the striatum by way of the thalamus, and may influence the pathways involved in basal ganglia processing (26). Neuroimaging studies suggested that the cerebellum has a role in accommodation to pathophysiology of PD via cerebello-thalamo-cortical circuits (27,28). Functional neuroimaging studies demonstrated increased activation in the cerebellum in patients with PD during performance of various upper limb movements (29,30). The nature of the hyperactivation of cerebellum in PD remains unclear and one likely explanation is that this phenomenon presents a compensatory effect.
This compensatory effect of cerebellum may be important in the levodopa treatment of parkinsonism of PD. Previous study compared motor activation in MSA and PD. It reported that PD preferentially activated cerebellar pathways after an acute levodopa challenge, possibly to compensate for basal ganglia dysfunction (31). This was not observed in MSA patients, probably because of cerebellar dysfunction (31). The cerebellum dysfunction explained, at least partly, the unresponsiveness of levodopa in patients with MSA. Similarly, our study found hypoactivation in the bilateral cerebellum during finger-tapping task and none responded to levodopa at baseline, which was in accordance with the pathophysiology of MSA.
After rTMS treatment, our study discovered significantly increased activation in the cerebellum in the EP group, which was accompanied by the improvement of UMSARS-II score. Although no significant correlation was identified between the increase of cerebellum activation and decrease of UMSARS-II score, the whole-brain connectivity analysis of resting-state fMRI data based on the same patient population did show that the motor symptom improvement in the active rTMS group was associated with positive changes in the cerebellar connectivity following the rTMS treatment (32). Therefore, our study revealed that the increased cerebellar activation was motor-effect-relevant in MSA, probably due to the cerebellar loop compensation induced by 5 Hz rTMS treatment. Future studies should make further investigation of the correlation between brain activity changes and improvement of clinical symptoms. The increased cerebellum activation may be the remote effect of rTMS and the mechanism of it was beyond the purpose of the current study.
Much attention should be paid to the fact that these patients were concurrently exposed to levodopa therapy. As the previously mentioned study suggested, the cerebellar functiona related to the different levodopa responsiveness of PD and MSA (31). However, it is unclear whether the recovery of cerebellar dysfunction may lead to the responsiveness of levodopa in MSA. The exposure to levodopa in this study could influence the nature of the after-effects (33). We could not confirm that it was purely rTMS effect rather than the combination effects of levodopa and rTMS. Thus our findings can’t be generalized to dopamine naive patients. Previous study in stroke postulated that the rTMS treatment could prepare the brain and enhance its response to other treatments (34). Based on that theory, we hypothesized that rTMS might accommodate the brain function, such as the adjustment of cerebellum dysfunction, to the levodopa treatment in patients with MSA.
There are several shortcomings in this study. First, we did not use the traditional sham stimulation. Second, the sample size was relatively small. Lastly, the levodopa exposure was not controlled. Further research is warranted to better specify the underlying mechanisms of rTMS effects and the interaction between rTMS and pharmacological treatment with an enlarged population.
In conclusion, the present study supports our hypotheses that 10-session 5 Hz rTMS over the left M1 could induce a significant improvement of Parkinsonism in MSA patients and a motor-effect-relevant increased activation in the cerebellum. Given that MSA is an uncontrollable disease, any potentially effective therapy raises interest and encourages further investigations.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation (30800352 to H Wang); the Science Technology and Innovation Committee of Shenzhen (JC201005270293A and JC201104220257A to Y Qiu); and the Hundred Talents Program of Chinese Academy of Sciences Grant (Y14408 to Y Qiu).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670-6. [Crossref] [PubMed]
- Wenning GK, Geser F, Krismer F, et al. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol 2013;12:264-74. [Crossref] [PubMed]
- Ozawa T, Healy DG, Abou-Sleiman PM, et al. The alpha-synuclein gene in multiple system atrophy. J Neurol Neurosurg Psychiatry 2006;77:464-7. [Crossref] [PubMed]
- Wenning GK, Tison F, Ben Shlomo Y, et al. Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord 1997;12:133-47. [Crossref] [PubMed]
- Ahmed Z, Asi YT, Sailer A, et al. The neuropathology, pathophysiology and genetics of multiple system atrophy. Neuropathol Appl Neurobiol 2012;38:4-24. [Crossref] [PubMed]
- Chou YH, Hickey PT, Sundman M, et al. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol 2015;72:432-40. [Crossref] [PubMed]
- Benninger DH, Hallett M. Non-invasive brain stimulation for Parkinson's disease: Current concepts and outlook 2015. NeuroRehabilitation 2015;37:11-24. [Crossref] [PubMed]
- Pascual-Leone A, Tormos JM, Keenan J, et al. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol 1998;15:333-43. [Crossref] [PubMed]
- Benninger DH, Berman BD, Houdayer E, et al. Intermittent theta-burst transcranial magnetic stimulation for treatment of Parkinson disease. Neurology 2011;76:601-9. [Crossref] [PubMed]
- George MS, Lisanby SH, Sackeim HA. Transcranial magnetic stimulation: applications in neuropsychiatry. Arch Gen Psychiatry 1999;56:300-11. [Crossref] [PubMed]
- Slotema CW, Blom JD, Hoek HW, et al. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry 2010;71:873-84. [Crossref] [PubMed]
- Ni Z, Chen R. Transcranial magnetic stimulation to understand pathophysiology and as potential treatment for neurodegenerative diseases. Transl Neurodegener 2015;4:22. [Crossref] [PubMed]
- Pascual-Leone A, Valls-Solé J, Brasil-Neto JP, et al. Akinesia in Parkinson's disease. II. Effects of subthreshold repetitive transcranial motor cortex stimulation. Neurology 1994;44:892-8. [Crossref] [PubMed]
- Khedr EM, Rothwell JC, Shawky OA, et al. Effect of daily repetitive transcranial magnetic stimulation on motor performance in Parkinson's disease. Mov Disord 2006;21:2201-5. [Crossref] [PubMed]
- Lomarev MP, Kanchana S, Bara-Jimenez W, et al. Placebo-controlled study of rTMS for the treatment of Parkinson's disease. Mov Disord 2006;21:325-31. [Crossref] [PubMed]
- Wenning GK, Tison F, Seppi K, et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov Disord 2004;19:1391-402. [Crossref] [PubMed]
- Geser F, Wenning GK, Seppi K, et al. Progression of multiple system atrophy (MSA): a prospective natural history study by the European MSA Study Group (EMSA SG). Mov Disord 2006;21:179-86. [Crossref] [PubMed]
- Sabatini U, Boulanouar K, Fabre N, et al. Cortical motor reorganization in akinetic patients with Parkinson's disease: a functional MRI study. Brain 2000;123:394-403. [Crossref] [PubMed]
- Mostofsky SH, Rimrodt SL, Schafer JG, et al. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biol Psychiatry 2006;59:48-56. [Crossref] [PubMed]
- Gountouna VE, Job DE, McIntosh AM, et al. Functional Magnetic Resonance Imaging (fMRI) reproducibility and variance components across visits and scanning sites with a finger tapping task. Neuroimage 2010;49:552-60. [Crossref] [PubMed]
- Jäncke L, Lutz K, Koeneke S. Converging evidence of ERD/ERS and BOLD responses in motor control research. Prog Brain Res 2006;159:261-71. [Crossref] [PubMed]
- Talairach P, J Tournoux. A Stereotactic Coplanar Atlas of the Human Brain. 1988.
- Friston KJ, Frith CD, Turner R, et al. Characterizing evoked hemodynamics with fMRI. Neuroimage 1995;2:157-65. [Crossref] [PubMed]
- Shulman LM, Gruber-Baldini AL, Anderson KE, et al. The clinically important difference on the unified Parkinson's disease rating scale. Arch Neurol 2010;67:64-70. [Crossref] [PubMed]
- Ghez C, Fahn S. The cerebellum. In: Kandel ER, Schwartz JH, editors. Principles of neural science. New York: Elsevier, 1985:502-22.
- Wu T, Hallett M. The cerebellum in Parkinson's disease. Brain 2013;136:696-709. [Crossref] [PubMed]
- Sen S, Kawaguchi A, Truong Y, et al. Dynamic changes in cerebello-thalamo-cortical motor circuitry during progression of Parkinson's disease. Neuroscience 2010;166:712-9. [Crossref] [PubMed]
- Wu T, Hallett M. Reply: The cerebellum in Parkinson's disease and parkinsonism in cerebellar disorders. Brain 2013;136:e249. [Crossref] [PubMed]
- Wu T, Wang L, Hallett M, et al. Neural correlates of bimanual anti-phase and in-phase movements in Parkinson's disease. Brain 2010;133:2394-409. [Crossref] [PubMed]
- Wu T, Hallett M. Neural correlates of dual task performance in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 2008;79:760-6. [Crossref] [PubMed]
- Payoux P, Brefel-Courbon C, Ory-Magne F, et al. Motor activation in multiple system atrophy and Parkinson disease: a PET study. Neurology 2010;75:1174-80. [Crossref] [PubMed]
- Chou YH, You H, Wang H, et al. Effect of Repetitive Transcranial Magnetic Stimulation on fMRI Resting-State Connectivity in Multiple System Atrophy. Brain Connect 2015;5:451-9. [Crossref] [PubMed]
- Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci 2007;8:559-67. [Crossref] [PubMed]
- Avenanti A, Coccia M, Ladavas E, et al. Low-frequency rTMS promotes use-dependent motor plasticity in chronic stroke: a randomized trial. Neurology 2012;78:256-64. [Crossref] [PubMed]


