Looking forward lung metastasectomy—do we need a staging system for lung metastases?
In the last decennium we are witnessing an impressive increase number of publications on lung metastasectomy (LM) ranging from commentaries, case series, comparative studies, reviews and systematic reviews as demonstrated in Figure 1.
The most common denominator between surgical papers is the tentative to demonstrate that LM may offer a prolonged survival. Moreover, the in deep review of the extensive literature demonstrated that the level of evidence to perform LM is weak and still today surgeons operated on the basis of their own experience (1-3).
The uncertainty on LM are several, and many questions remain unresolved. For example, the progression of metastasis is still not well known but it seems that it is mainly due to a small fraction of tumor cells with the capability to navigate from primary tumor cell to end-organ metastasis. For this reason it is possible that in the future the “so called” liquid biopsy to discover circulating tumour DNA (ctDNA) could detect the presence of ctDNA in the blood of patients with LM burden (4).
Unfortunately, most patients with pulmonary metastases will not be candidates for LM because the presence of comorbidities or the need of extended surgery (5,6). In these patients new approaches are available such as radiotherapy, radiofrequency and microwave ablation, and immunotherapy, but these treatments are in search of evidence (7). Recently, new strategies have been reported to obtain long term survival such as VATS lung suffusion, and a phase I trial isolating cisplatin for 30 minutes to the side of metastasectomy or primary lung cancer with oligometastases is underway (8).
As regards surgery, the indication for surgery in LM, oligometastatic or not, has a deep impact in the patient mood because the patients think that it is possible to eradicate the disease, and therefore to live longer (9,10). A recent survey in Spain demonstrated the presence of oligometastatic disease in 55% of patients (11). But some patients with a “resectable” metastatic disease in the lung will not survive longer as expected, and therefore the final results is that the practical operation to remove “resectable” disease is not the right operation which should have been performed. The obvious question is if it is ethical to operate a patient with “resectable” disease informing her/him that we have no prove that survival will be longer compared to the “no” operation group (12). In general, patients with lung metastases should be fit enough to undergo surgery, the primary disease site and any extrathoracic disease should be both controlled; complete resection of pulmonary involvement should be achievable with adequate pulmonary reserve; and no effective medical therapies should exist (13). Regarding the type of surgery, several techniques have been proposed to perform unilateral or bilateral LM removing one or multiple metastases. Moreover few studies have demonstrated that no difference in survival exists between open surgery and VATS when LM needs to be removed (14-16). For this reason it is wise to conclude that the less invasive approach should be used, until proved differently. Furthermore another review reported that prolonged long term survival in LM of colorectal cancer is obtainable only in patients with single metastasis (17). Although some authors first reported that repeated metastasectomy could prolong survival, successively reviewing again the data they think that better survival attributed to LM may be due to the selection of patients who are innately destined to live longer (18,19).
During surgery, a positive node N1 or N2 adversely affects survival in LM, and the incidence of nodal metastases has an estimate of 20% to 25% across multiple tumor types (8). Although there is no definitive answer, we could speculate that nodal dissection should be performed mainly to stage accurately the disease than to prolong survival. In another hand, pre-operative suspicion of lymph node involvement should also be investigated actively by mediastinal procedures (EBUS, mediastinoscopy, VATS) to confirm histologically the invasion to avoid unnecessary surgery (20).
The presence or absence of nodal metastases in LM is therefore an important point, and stimulates a question. Do we need a staging system for lung metastases? A staging system which focuses on lung metastases with the main AIM to categorize our patients, and predict survival is probably necessary. An attempt of staging system for lung metastases is shown and explained in Table 1. Time to metastasis (TM), defined as the time of appearance of lung metastasis, could also be added in the staging system to separate early from late lung metastases with an e (early) before the TNM, so that eTNM means an early appearance of lung metastases.
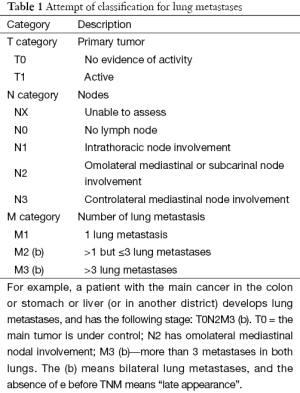
Full table
Finally, although the action of metastasectomy could be criticized as it could be seen as an overtreatment, on the other side it is difficult to explain to our patients that surgical resection of LM to prolong survival is in doubt. Should we deny this “hope” to our patients? Certainly not, we agree with some authors (3) that surgery is the current available hope that our patients have, and although there is the increase feeling of unfounded believe of longer survival with LM, we need to offer them the best treatment available.
Surgical opinion based on our own experience, but not on data, should not be the right approach anymore, and for this reason few years ago we joined the PulMiCC trial international (21). The PulMiCC trial, a vision of Tom Treasure, was initiated in UK in 2010 (22), after few years some European centers in Serbia and Italy joined the trial (23,24), which is now also open in China. Nowadays, technology permits a worldwide cooperation which will permit to collect data faster, and consequently large randomized controlled studies could be finish. The PulMiCC trial is representing an innovative worldwide scientific group with specific interest in LM to definitively answer if metastasectomy should be performed in patients with lung metastasis of colorectal cancer.
Acknowledgements
M Migliore had the idea, designed and wrote the first draft of the manuscript; M Gonzalez agreed and contributed in writing. M Migliore and M Gonzalez approved the final version of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Treasure T, Milošević M, Fiorentino F, et al. Pulmonary metastasectomy: what is the practice and where is the evidence for effectiveness? Thorax 2014;69:946-9. [Crossref] [PubMed]
- Van Raemdonck D. Pulmonary metastasectomy: common practice but is it also best practice? Future Oncol 2015;11:11-4. [Crossref] [PubMed]
- Mineo TC, Ambrogi V. Lung metastasectomy: an experience-based therapeutic option. Ann Transl Med 2015;3:194. [PubMed]
- The Lancet Oncology. Liquid cancer biopsy: the future of cancer detection? Lancet Oncol 2016;17:123. [Crossref] [PubMed]
- Erhunmwunsee L, Tong BC. Preoperative Evaluation and Indications for Pulmonary Metastasectomy. Thorac Surg Clin 2016;26:7-12. [Crossref] [PubMed]
- Migliore M, Jakovic R, Hensens A, et al. Extending surgery for pulmonary metastasectomy: what are the limits? J Thorac Oncol 2010;5:S155-60. [Crossref] [PubMed]
- Macbeth F, Treasure T. Stereotactic Ablative Radiotherapy for 'Oligometastases': a Treatment in Search of Evidence. Clin Oncol (R Coll Radiol) 2016. [Epub ahead of print].
- Demmy TL. Thoracoscopic Lung Suffusion. Thorac Surg Clin 2016;26:109-21. [Crossref] [PubMed]
- Treasure T, Macbeth F. Is Surgery Warranted for Oligometastatic Disease? Thorac Surg Clin 2016;26:79-90. [Crossref] [PubMed]
- Treasure T, Macbeth F, Russell C. If no difference in effectiveness is found between two treatments it may be because the treatments are similarly ineffective. Ann Transl Med 2015;3:201. [PubMed]
- Embún R, Fiorentino F, Treasure T, et al. Pulmonary metastasectomy in colorectal cancer: a prospective study of demography and clinical characteristics of 543 patients in the Spanish colorectal metastasectomy registry (GECMP-CCR). BMJ Open 2013.3. [PubMed]
- Migliore M. Comment on Godlee F. Colorectal Cancer: a cautionary tale. Available online: http://www.bmj.com/content/348/bmj.g3311/rr/778360
- Downey RJ, Bains MS. Open Surgical Approaches for Pulmonary Metastasectomy. Thorac Surg Clin 2016;26:13-8. [Crossref] [PubMed]
- Migliore M, Criscione A, Calvo D, et al. Wider implications of video-assisted thoracic surgery versus open approach for lung metastasectomy. Future Oncol 2015;11:25-9. [Crossref] [PubMed]
- Greenwood A, West D. Is a thoracotomy rather than thoracoscopic resection associated with improved survival after pulmonary metastasectomy? Interact Cardiovasc Thorac Surg 2013;17:720-4. [Crossref] [PubMed]
- Perentes JY, Krueger T, Lovis A, et al. Thoracoscopic resection of pulmonary metastasis: current practice and results. Crit Rev Oncol Hematol 2015;95:105-13. [Crossref] [PubMed]
- Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:572-9. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Tacconi F, et al. Multi-reoperations for lung metastases. Future Oncol 2015;11:37-41. [Crossref] [PubMed]
- Treasure T, Mineo T, Ambrogi V, et al. Survival is higher after repeat lung metastasectomy than after a first metastasectomy: Too good to be true? J Thorac Cardiovasc Surg 2015;149:1249-52. [Crossref] [PubMed]
- Call S, Rami-Porta R, Embún R, et al. Impact of inappropriate lymphadenectomy on lung metastasectomy for patients with metastatic colorectal cancer. Surg Today 2016;46:471-8. [Crossref] [PubMed]
- Migliore M, Lees B, Treasure T, et al. Randomized controlled trial of pulmonary metastasectomy in colorectal cancer: PulMiCC International is open in Italy. Oncologist 2013;18:637. [Crossref] [PubMed]
- Treasure T, Fallowfield L, Lees B. Pulmonary metastasectomy in colorectal cancer: the PulMiCC trial. J Thorac Oncol 2010;5:S203-6. [Crossref] [PubMed]
- Migliore M, Milošević M, Lees B, et al. Finding the evidence for pulmonary metastasectomy in colorectal cancer: the PulMicc trial. Future Oncol 2015;11:15-8. [Crossref] [PubMed]
- Treasure T, Miloševic M, Migliore M, et al. Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC International). Colorectal Cancer 2013;2:505-13. [Crossref]



