Long-term survival with repeated resections of recurrent hepatocellular carcinoma in a non-cirrhotic liver: case report and brief review of the literature
Introduction
Hepatocellular carcinoma (HCC) is the commonest primary hepatic malignancy, which in 10–20% of cases is diagnosed in the absence of cirrhosis. In non-cirrhotic HCC, hepatic resection is currently the preferred treatment, with a reported perioperative mortality and morbidity of 0–6% and 8–40% respectively (1,2). Unfortunately, these tumors tend to recur both in the early (<1 year), as well as in the late (≥1 year) postoperative period (3). In the following report we present a case of an otherwise healthy and asymptomatic woman who underwent a complete (R0) non-anatomical hepatic resection for HCC, followed by two additional R0 resections for recurrence at 6 and at 12.5 years. To our knowledge, this pattern of long-term recurrences in the absence of background cirrhosis is very infrequent. With this report we aim to raise awareness of the propensity for late HCC recurrences in patients with normal background liver parenchyma, and to raise the question of the optimal surveillance strategy and genetic investigations.
Case presentation
An otherwise healthy 55-year-old female office worker was referred to our Hepatopancreatobiliary (HPB) Department by her General Practitioner (GP) with an incidental asymptomatic right-upper-quadrant mass. Her medical history was unremarkable, and in particular did not include hepatitis, jaundice, constitutional symptoms suggestive of malignancy, or chemical exposure. Drug history was notable for previous oral contraceptive pill use, followed by two short episodes of hormone replacement therapy (HRT). Family history was notable for breast cancer only, and the patient was an ex-smoker of 15 pack-years with an alcohol intake of 5–10 UK units per week.
General physical examination was unremarkable, without stigmata of chronic liver disease or lymphadenopathy, and abdominal examination confirmed a palpable smooth right-upper-quadrant mass without ascites.
Investigations
Ultrasonography (US) suggested a solitary liver tumor, which was characterized by computed tomography (CT) and magnetic resonance imaging (MRI) as an 8 cm Segment 5 (SgV) HCC in a non-cirrhotic liver. Full blood count (FBC) and liver function test (LFT) values were normal, with the exception of a mildly deranged serum bilirubin of 19 µmol/L (normal range, <17 µmol/L). Serum tumor marker testing showed normal CEA and CA19-9 levels, an elevated βHCG at 10 IU/L (normal range, <5 IU/L) and a markedly increased αFP of 4,203 ng/mL (normal range, <5.52 ng/mL).
Treatment, outcome and follow-up
The patient underwent an uneventful laparoscopic wedge resection of the SgV lesion with concurrent cholecystectomy. Histopathological analysis of the surgical specimen confirmed an 8.5-cm moderately-to-well differentiated HCC without lymphatic or vascular invasion, completely resected (R0) with a clear rim of normal parenchyma ranging between 7 and 33 mm. No adjuvant treatment was instituted, and the patient underwent biannual follow-up for the next six years, during which αFP levels fluctuated between 9 and 12 ng/mL, without clinico-radiological evidence of recurrence. Two years postoperatively the patient was diagnosed with node-negative invasive lobular carcinoma of the left breast and underwent wide local excision followed by radiotherapy, docetaxel and tamoxifen.
At six years post initial HCC diagnosis, surveillance CT demonstrated a 1.9-cm SgIVa recurrent intrahepatic HCC with a concomitant serum αFP level of 40.8 ng/mL. The patient underwent an uneventful SgIV wedge resection and histology demonstrated a moderately differentiated HCC with clear resection margins. An additional SgVIII lesion identified intraoperatively on intraoperative US was also resected but this was found negative for malignancy on histology (Figure 1).
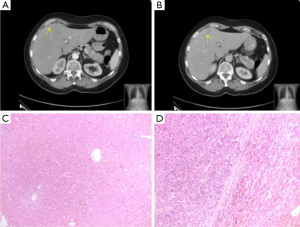
During the ensuing seven years following the second liver resection, the patient was followed up biannually with reassuring imaging results, and αFP levels which fluctuated between 9 and 16 ng/mL. After this period of time (12.5 years post initial diagnosis), a 2-cm SgII/SgIII recurrent intrahepatic HCC was identified on both CT and MRI, with a corresponding αFP value of 47 ng/mL. At this point the patient underwent her third operation, an uneventful open left lateral hepatectomy with histology confirming R0 resection of HCC with vascular invasion (Figure 2).
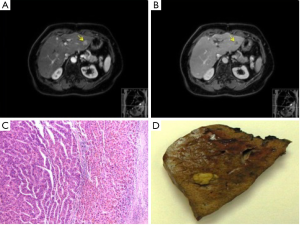
At 2 years following the latest procedure (14 years and 7 months after initial resection), the patient remained asymptomatic without surgical complications. She is on 6-monthly active surveillance, with imaging which has excluded recurrence and metastasis, corresponding with normal αFP levels.
Discussion
HCC is the commonest primary hepatic malignancy with an annual global incidence estimated at 700,000 cases, which is steadily increasing. HCC is the 5th most frequently diagnosed cancer and the 3rd leading cause of cancer deaths (4,5). HCC develops on a background of cirrhosis in nearly 90% of cases, with a male-to-female ratio between 2:1 and 4:1 (5,6). Conversely, the incidence of HCC in the non-cirrhotic liver (HCC-NCL) is between 15–20%, and that of HCC in the normal liver (HCC-N) is between 10–12% (7).
Etiology & pathogenesis
HCC is a remarkably heterogeneous entity, both at the molecular and at the clinical level, with a variety of incompletely understood pathogenetic mechanisms. Similarly, “non-cirrhotic liver” cohorts are heterogeneous as they include patients with viral and non-viral hepatitis, steatosis, fibrosis, and exposure to a variety of risk factors, as discussed below.
Viral infection is one of the main risk factors for the development of HCC-NCL. HBV genome integration can lead to DNA microdeletions and genotoxic product formation, altering transcription and leading to HCC even in the absence of cirrhosis. HCV infection on the other hand promotes hepatic necro-inflammation and leads to HCC mainly via cirrhosis (2). Hepatocarcinogenic substances implicated in HCC-NCL include alcohol, aflatoxin B1, various industrial agents, and thorium. Additionally, HCC-NCL may result from inherited conditions such as hemochromatosis, glycogen storage disease, congenital hepatic fibrosis and Budd Chiari syndrome to name only a few (2). The genetic alterations leading to HCC in a normal (i.e., non-cirrhotic, non-steatotic, non-fibrotic, seronegative) liver remain to be fully elucidated by next-generation exome sequencing (8). In contrast to HCC with cirrhosis (HCC-C) which is related to p53 expression alterations and activation of the Wnt/β-catenin pathway, HCC-NCL is more associated with cell cycle disruption via β-catenin mutations, alterations in p14, p21 and p27 expression, as well as gene methylation (9,10). For the minority of patients with HCC-N, malignant transformation of hepatocellular adenoma has been implicated, as well as CTNNB1 and TERT promoter mutations (8).
In the current case, the only obvious potential etiology is previous contraceptive use and HRT. Estrogen in particular, has been linked to an increased risk of hepatic cancer via its action on initiators and promoters of carcinogenesis, as well as by induction of vascular changes or tumor cell hypertrophy (2).
Clinico-pathological features
Although the natural history of HCC-NCL is poorly understood, several patterns of clinical presentation have been recognized (7):
- The classic form, whereby patients present with pain and a large tumor (8–10 cm)
- The tumor rupture with hemorrhage form
- The massive late-stage form, which usually affects younger patients and is characterized by distant metastases
- The asymptomatic form
In a multicentric analysis of 334 patients with HCC-NCL, Arnaoutakis et al. (9) found a median patient age of 58 years and a male predilection at 77%. Tumors were predominantly solitary (81%) as in the current case, with poor or no differentiation in 56% of cases, and a median size of 6.5 cm (range, 1.1–40.2 cm). The majority of livers were non-fibrotic (82.5%), non-steatotic (80.6%), and nearly half of all patients (48.8%) were seronegative for hepatitis B & C. Only 12.6% of patients had a history of alcohol abuse. Presenting features in order of decreasing frequency were abdominal pain (53%), early satiety (15%), weight loss (12%), and an abdominal mass (6%). Although most (93.4%) patients underwent resection, this cohort also included cases of embolization, chemotherapy, ablation and liver transplantation (LT) (9).
In a more homogeneous, albeit smaller cohort, Laurent et al. (6) analyzed 108 cases of HCC-NCL, all of which were resected. The investigators found an average age of 64 years, and a male predilection at 82%. Tumors were solitary in 80% of cases, with a mean size of 9.3 cm (range, 1.0–25.0 cm). Fifty percent of patients consumed alcohol excessively, 38% used tobacco, 37% had a BMI ≥27 and 20% were diabetic (6).
Lubrano et al. (11) focused on a cohort of 20 patients with HCC-N, treated by resection. Patients were again predominantly male (70%), with a median age of 57 and presented with either abdominal pain, a palpable tumor, weight loss, or jaundice. The median tumor diameter was 9.0 cm (range, 3.0–17.0 cm) and most (80%) tumors were solitary (11).
Taking a different approach, Young et al. (12) compared 81 patients with HCC-N to 61 patients with HCC in “diseased” liver (i.e., with any of the following: cirrhosis, fibrosis, chronic liver disease, steatohepatitis, viral hepatitis), all of whom underwent resection. As part of this study, patients with mild-to-moderate steatosis without hepatitis were considered to have HCC-N. Univariate analysis found that HCC-N patients were more likely to present with larger (≥10 cm) solitary tumors vs. patients with HCC in diseased liver (12). HCC-N patients presented with pain (44%), after incidental diagnosis (16%), with a palpable mass (12%), malaise (8%), weight loss (8%), liver function derangement (6%), tumor rupture (4%), nausea (2%), or dyspnea (2%) (12).
More recently, Schütte et al. (13) compared 93 patients with HCC-NCL to 571 patients with HCC-C, and found that HCC-NCL affected more women (27% vs. 18%) and was diagnosed at an older age (69 vs. 66 years), with better liver synthetic and biochemical profiles. Furthermore, HCC-NCL lesions were larger (9.1 vs. 6.5 cm), and presented with extrahepatic metastases more frequently (37.21% vs. 25.79%), but less frequently with ascites (20.24% vs. 44.30%) (13).
A metanalysis by Zhou et al. (14), which included 1,333 patients with HCC-NCL and 1,980 patients with HCC-C, confirmed that HCC-NCL patients present with better liver function, larger tumors and more advanced disease. However, these authors found no significant differences in gender or age (14). It should be noted that the study by Schütte et al. (13) was not included in this metanalysis.
Postoperative outcomes & prognosis
HCC-NCL can be treated non-operatively (e.g., by radiofrequency or microwave ablation, percutaneous ethanol injection, transarterial chemoembolization, and systemic chemotherapy), operatively (by liver resection or transplantation), or by a combination of the above (5). Liver resection is currently the treatment of choice, offering relatively good short-term and long-term outcomes. Specifically, a systematic review of 3,771 HCC-NCL cases demonstrated that resection confers postoperative morbidity and mortality risks of 29.5% and 2.7% respectively, with 5-year OS and DFS rates of 47.9% and 38.0% respectively (14). These postoperative mortality, OS, and DFS rates are in fact superior when compared to HCC-C cases. Postoperative morbidity rates however, are similar (14).
LT is not routinely recommended as it carries a higher risk and cost vs. resection (14), and also commits patients to lifelong immunosuppression. Patients with HCC and cirrhosis are usually selected for LT on the basis of the Milan criteria (one tumor ≤5 cm, or ≤3 tumors each measuring ≤3 cm). In their analysis of the literature and European Liver Transplant Registry, Mergental & Porte (15) identified more than 150 HCC-NCL patients who underwent LT, achieving a 5-year survival rate of 50–70%. Although macrovascular invasion, hilar lymphadenopathy, and the presence of multiple tumors were recognized as prognostic factors, tumor diameter was found to be irrelevant. The authors therefore concluded that LT should be considered in unresectable HCC-NCL, and that the Milan criteria are not applicable in these cases (15). This view was subsequently echoed in an international consensus report. The consensus panel also recommended that HCC-NCL patients with post-resection intrahepatic recurrence, without macrovascular invasion or extrahepatic disease, should be considered for salvage LT (16).
Recurrence of HCC-NCL following resection is common, with 1- and 5-year recurrence-free survival (RFS) rates quoted at 71.15% and 35% respectively (9). RFS in HCC-NCL appears to be negatively affected by HBV infection, poor tumor differentiation, macrovascular invasion, satellitosis, the presence of intrahepatic metastases, intraoperative blood transfusion, and incomplete resection (6,7,9,17). In the event of recurrence, an aggressive strategy of repeat resection has been associated with a 67% 5-year survival rate (17). In the current case, the initial and recurrent tumors were indeed resectable. Although our patient met the Milan criteria in terms of tumor size and number on each recurrence, repeat resection was deemed to be both the safest and most effective approach, leaving the patient with adequate healthy liver parenchyma. At 12.5 years (150 months) following initial resection, this case represents one of the longest periods between initial presentation and latest recurrence of HCC-NCL to be reported. To our knowledge, the longest recurrence period reported is 155 months (17). Such very late recurrences, combined with the fact that adjuvant treatment has not yet been established (7), warrant routine long-term (and perhaps life-long) surveillance. The challenge however lies in defining the duration, eligibility and therefore cost-effectiveness of such a surveillance program.
In conclusion, this case of very late HCC recurrence in a normal liver, following repeated complete resections, raises the issues of genetic screening, prognostication, and surveillance duration. It is hoped that advances in next-generation sequencing, alongside family cluster studies and long-term follow-up will clarify the molecular pathogenesis and perhaps reveal specific therapeutic targets.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Faber W, Seehofer D, Neuhaus P, et al. Repeated liver resection for recurrent hepatocellular carcinoma. J Gastroenterol Hepatol 2011;26:1189-94. [Crossref] [PubMed]
- Trevisani F, Frigerio M, Santi V, et al. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis 2010;42:341-7. [Crossref] [PubMed]
- Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 2015;261:947-55. [Crossref] [PubMed]
- Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 2014;63:844-55. [Crossref] [PubMed]
- Khan AS, Fowler KJ, Chapman WC. Current surgical treatment strategies for hepatocellular carcinoma in North America. World J Gastroenterol 2014;20:15007-17. [Crossref] [PubMed]
- Laurent C, Blanc JF, Nobili S, et al. Prognostic factors and longterm survival after hepatic resection for hepatocellular carcinoma originating from noncirrhotic liver. J Am Coll Surg 2005;201:656-62. [Crossref] [PubMed]
- Alkofer B, Lepennec V, Chiche L. Hepatocellular cancer in the non-cirrhotic liver. J Visc Surg 2011;148:3-11. [Crossref] [PubMed]
- Nault JC. Pathogenesis of hepatocellular carcinoma according to aetiology. Best Pract Res Clin Gastroenterol 2014;28:937-47. [Crossref] [PubMed]
- Arnaoutakis DJ, Mavros MN, Shen F, et al. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: a multi-institutional analysis. Ann Surg Oncol 2014;21:147-54. [Crossref] [PubMed]
- Tretiakova MS, Shabani-Rad MT, Guggisberg K, et al. Genomic and immunophenotypical differences between hepatocellular carcinoma with and without cirrhosis. Histopathology 2010;56:683-93. [Crossref] [PubMed]
- Lubrano J, Huet E, Tsilividis B, et al. Long-term outcome of liver resection for hepatocellular carcinoma in noncirrhotic nonfibrotic liver with no viral hepatitis or alcohol abuse. World J Surg 2008;32:104-9. [Crossref] [PubMed]
- Young AL, Adair R, Prasad KR, et al. Hepatocellular carcinoma within a noncirrhotic, nonfibrotic, seronegative liver: surgical approaches and outcomes. J Am Coll Surg 2012;214:174-83. [Crossref] [PubMed]
- Schütte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol 2014;14:117. [Crossref] [PubMed]
- Zhou Y, Lei X, Wu L, et al. Outcomes of hepatectomy for noncirrhotic hepatocellular carcinoma: a systematic review. Surg Oncol 2014;23:236-42. [Crossref] [PubMed]
- Mergental H, Porte RJ. Liver transplantation for unresectable hepatocellular carcinoma in patients without liver cirrhosis. Transpl Int 2010;23:662-7. [Crossref] [PubMed]
- Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:e11-22. [Crossref] [PubMed]
- Bège T, Le Treut YP, Hardwigsen J, et al. Prognostic factors after resection for hepatocellular carcinoma in nonfibrotic or moderately fibrotic liver. A 116-case European series. J Gastrointest Surg 2007;11:619-25. [Crossref] [PubMed]


