
Establishment of a patient-derived organoid model and living biobank for nasopharyngeal carcinoma
Introduction
Nasopharyngeal carcinoma (NPC) originates from nasopharynx epithelial cells and is common in southern China and Southeast Asia (1-3). NPC is causally linked to Epstein-Barr virus (EBV) infections (4,5). The 5-year overall survival rate for NPC is above 80% (6,7). However, recurrence occurs in 10% to 15% of patients with NPC, and the 5-year overall survival rate for patients with recurrent NPC is only 13.2% to 38% (8,9). The high rate of recurrence is responsible for the majority of failed NPC treatments with unfavorable prognosis (10), and tumor heterogeneity might be a cause of tumor recurrence (11,12).
Currently, NPC cell lines are the most commonly used model of NPC study. However, many NPC cell lines were reported to be contaminated by HeLa cell (13). Moreover, EBV viral genome was observed to be lost during long-term culture in most NPC cells (14,15). Most importantly, cell lines do not reflect the molecular heterogeneity of clinical NPC tissues (16). As a revelational disease model, 3-dimensional (3D) organoids are based on high hopes in oncology studies. Organoids maintain phenotypic and molecular heterogeneity of their parental tissue, be for with minor genetic aberration or transformation extended periods cultured in vitro (17,18). The lack of a living model is a factor limiting the study of relapsed and refractory NPC. Therefore, the establishment of a recurrent NPC biobank using the patient-derived organoid model is of significance to clinical and experimental studies.
In this study, we optimized technological process of patient-derived NPC organoids (NPCOs) modeling, and raised the success rate to more than 80%. For the first time, we established a living biobank/database of, which will provide powerful tools for high throughput research and drug development of recurrent nasopharyngeal carcinoma. We present the following article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1076/rc).
Methods
Ethical approval and sample collection
All the tissue samples were obtained from the Nanfang Hospital of the Southern Medical University (Guangzhou, China). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Biopsy and surgical resection samples were collected after written informed consent forms were obtained from the patients, and all related procedures were performed with the approval of the Research Ethics Committee at the Nanfang Hospital of the Southern Medical University (approval No. TCHIRB-10701103-E). Half of the NPC samples prepared for organoid generation were used for pathological experiments. After being washed with phosphate-buffered saline (PBS) containing 3% penicillin, streptomycin, and amphotericin B (Sigma), the NPC tissues used to generate the NPCOs were blotted dry and weighed. Each NPCO and NPC tissue was embedded in paraffin and sectioned at 4 µm for evaluation by hematoxylin and eosin (H&E), immunofluorescence (IF), immunohistochemistry (IHC), and EBV-encoded small RNA (EBER) in situ hybridization (ISH). Each section was independently evaluated by two pathologists.
Organoid culture, cryopreservation, and resuscitation
After thoroughly washing the NPC tissues with PBS, they were cut with scissors into 3 mm × 3 mm × 3 mm pieces and then digested in digestion buffer I (500 ng/mL hydrocortisone, 300 U/mL collagenase II, 100 U/mL hyaluronidase) at 37 ℃ and 50 rpm for 1.5 to 2 hours. This was followed by a second round of digestion within digestion buffer II (5 mg/mL Dispase II, 0.1 mg/mL DNase I). Large clumps and cell debris were removed using a 100-µm filter, after which the filtered cells were washed 3 times with ice-cold PBS. The NPC cells were resuspended using Matrigel at a density of 6×104 cells/mL. The cells were seeded into 6-well plates at 3 droplets per well and placed in an incubator at 37 ℃. The organoid culture medium was changed every 3 days. The Matrigel was disrupted by pipetting to passage the organoids. The organoids were digested using TrypLE (Life Technologies, cat. no. 12605-010) at 37 ℃ for 3 to 5 minutes. After centrifugation at 300 to 400 ×g for 2 minutes, the cell pellet was resuspended in Matrigel supplemented with organoid culture medium and plated in 30-µL droplets in a 6-well plate. When the Matrigel was polymerized, the organoids were cultured in the organoid culture medium at 37 ℃.
H&E staining
To validate whether NPCOs could reliably recapitulate the histopathological characteristics of NPC tissues, we performed an H&E staining assay using NPC tissues, NPCOs, and nasopharyngeal mucosa organoids (NMOs). Formalin-fixed paraffin-embedded (FFPE) slides from the tissues or organoids were subjected to routine H&E staining. The H&E-stained slides were independently assessed by two pathologists.
Immunohistochemistry
Paraffin-embedded sections were cut into 4-µm slices with a microtome (Leica RM2125 RTF). The slides were stained with primary antibodies against CK7 (1:200, Zhongshan Golden Bridge Biotechnology, Beijing China), BMI-1 (1:500, Zhongshan Golden Bridge Biotechnology), and negative control antibodies. Subsequently, the tissue sections were stained with DAKO (DAKO, K346711-2, Copenhagen, Denmark) liquid 3,3’-diaminobenzidine tetrahydrochloride (DAB) for 10 minutes, counterstained with 10% Mayer’s hematoxylin, dehydrated, and mounted. Each section was independently evaluated by 2 pathologists.
Immunofluorescence
Organoids were fixed with 10% formalin, permeabilized in Triton X-100 (Sigma-Aldrich, cat. no. 9036-19-5), and blocked with 5% bovine serum albumin (BSA) in PBS. The slides were incubated with primary antibodies against CD44 and CD133 (1:200, Affinity, Colorado, United States,) overnight, washed 3 times with PBS, and stained with the appropriate secondary antibody along with phalloidin (Thermo Fisher Scientific, Delaware, United States). The nuclei were counterstained using Hoechst (Thermo Fisher Scientific). All the samples were mounted using ProLong Gold (Thermo Fisher Scientific). Fluorescent cells were recorded and photographed using a Leica fluorescence microscope.
In situ hybridization
To determine EBV status, paraffin-embedded tissues underwent ISH using oligonucleotides complementary to EBER transcripts. An EBER ISH kit was used in accordance with the manufacturer’s instructions (Zhongshan Golden Bridge Biotechnology, Beijing, China). Appropriate negative and positive controls were used.
Statistical analysis
All data were represented as mean ± the standard deviation calculated from 3 independent experiments. P values <0.05 were considered statistically significant.
Results
Generation of NPCOs and NMOs
In January 2021, Professor Sasidharan Swarnalatha Lucky published an article saying that PDX-derived nasopharyngeal carcinoma organoids were established for the study of nasopharyngeal carcinoma radiotherapy (19). In June of the same year, Professor Deng publicized the establishment of nasopharyngeal carcinoma organoids for the study of the molecular mechanism of nasopharyngeal carcinoma (20). However, both organoids have the defects of high culture cost, complicated operation process and low success rate. In this study, we developed a improved protocol to generate NPCOs. NPC tissues were minced into small pieces with ophthalmic scissors. The pieces were digested and subsequently passed through a cell strainer. We found that Matrigel could help to generate NPCOs (Figure S1A). Previous studies had found that several cytokines, including Wnt3a, R-spondin-1 (Rspo-1), EGF, Y27632, Noggin, and FGF2, were important for organoid generation (21-24). To develop an optimized culture medium for NPCO, we detected the growth of NPCOs in conditioned media with different concentration gradients of each cytokine. We found that the optimal concentrations of Wnt3a, Rspo-1, EGF, Y27632, Noggin, and FGF2 were 250, 500, 5, 10, 500, and 5 ng/mL, respectively (Figure S1B,S1C).
After the NPC cells had been cultured for 24 hours, NPCOs were generated (Figure S2). We used 62 NPC samples, including 34 primary and 28 recurrent NPC tissue samples, to generate the NPCOs. Information regarding these NPC samples, including the method of tissue obtention, patient sex and age, clinical stage, disease characteristics, lesion location, tissue mass, and tissue ex vivo time, is summarized in Table 1. A total of 39 NPCOs were successfully generated with an overall efficiency of 62.9%. Of these 39 NPCOs, 16 were derived from primary NPC patients with no chemotherapy history, and 23 were derived from patients with recurrent NPC (Table 1). The success rates of primary and recurrent NPCO formation were 47.06% and 82.14%, respectively (P=0.004). For the 28 recurrent NPC samples, the success rates of organoid generation from primary and metastatic lesions were 87.5% and 50%, respectively (Table 1). The biopsy mass of the NPC tissues also affected the NPCO generation, which might have been due to the NPC cell numbers (P=0.009). The success rate of the NPCO generation was not affected by age or sex (P=0.916 and 0.114, respectively), but it was significantly influenced by the method used to obtain the tissue, disease characteristics, tissue mass, and tissue ex vivo time (P=0.0006, 0.004, 0.001, and 0.023, respectively) (Table 1).
Table 1
| Variables | Grouping | Cases | No. of successful cases | Success rate (%) | P value |
|---|---|---|---|---|---|
| Method of tissue obtention | Biopsy | 31 | 13 | 40 | 0.0006*** |
| Surgery | 31 | 26 | 83.87 | ||
| Sex | Male | 35 | 25 | 71.43 | 0.114 |
| Female | 27 | 14 | 51.85 | ||
| Age, years | ≤45 | 30 | 18 | 60 | 0.916 |
| 45–59 | 23 | 15 | 65.22 | ||
| >59 | 9 | 6 | 66.67 | ||
| Clinical stage | III | 32 | 20 | 62.5 | 0.944 |
| IV | 30 | 19 | 63.33 | ||
| Primary/recurrent NPC | Primary | 34 | 16 | 47.06 | 0.004** |
| Recurrent | 28 | 23 | 82.14 | ||
| Lesion location | Primary focus | 58 | 27 | 46.55 | 0.784 |
| Metastasis | 4 | 2 | 50 | ||
| Tissue mass | ≥50 mg | 49 | 38 | 77.08 | 0.001** |
| <50 mg | 13 | 1 | 3.22 | ||
| Tissue ex vivo time | ≤12 h | 35 | 25 | 71.43 | 0.023* |
| 12–24 h | 18 | 12 | 66.67 | ||
| 24–48 h | 9 | 2 | 22.22 |
Among them, 30 of 31 biopsy samples were obtained by endoscopy and only 1 case was from pleural metastases of nasopharyngeal carcinoma metastases. *, P<0.05; **, P<0.01; ***, P<0.001. NPCO, nasopharyngeal carcinoma organoid.
We then used nasopharyngeal mucosa specimens to establish NMOs. From 15 attempts, 13 NMOs were successfully generated. In contrast to the NPCOs, the NMOs presented 3 morphology types: regular vacuoles, irregular vacuoles, and irregular solid shapes (Figure 1A). The vacuolated NMOs were similar to the lumen of the nasopharyngeal mucosa gland, and their diameters ranged from 200 to 250 µm (Figure 1A). However, the NPCOs showed a regular spherical shape with a diameter of 150 to 200 µm (Figure 1A). H&E staining showed that the NMO cell morphology was consistent with that of its corresponding parent nasopharyngeal mucosa tissue (Figure 1B). NMOs, like nasopharyngeal mucosa tissues, were well-differentiated, and their cell and nucleus sizes were uniform (Figure 1B). However, the NPCO and NPC tissues were poorly differentiated in H&E staining (Figure 1C). Furthermore, IHC showed the NMOs to be CK7 positive, while the NPCOs were CK7 negative, consistent with the IHC results for NPC tissue (Figure 1D).
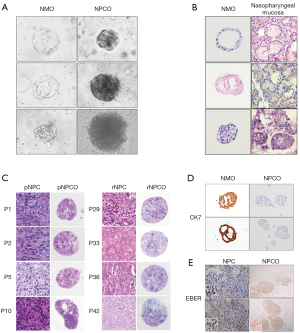
Almost 100% of NPC cases are associated with EBV infection. We performed EBER ISH assays and found that each NPCO and its parental NPC tissue were EBER positive (Figure 1E).
Overall, we successfully generated NMOs and NPCOs and found that the NPCOs could maintain the features of NPC tumors, including EBV infection status and clinical characteristics.
Composition of stem cell-derived NPCOs
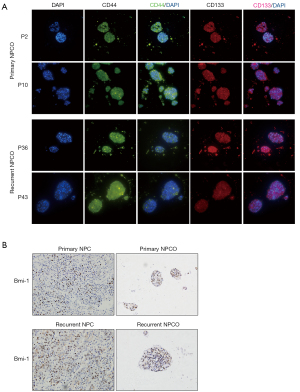
Previous studies have suggested that organoids be derived from stem cells and generated via special differentiation pluripotent stem cells (PSCs) (24,25). Most NPC tissues are poorly differentiated. We performed IF assays to detect the expression of typical stem cell markers, including CD44 and CD133. The IF results showed that CD44 and CD133 were widely distributed throughout the NPCOs, which indicated that the NPCOs were stem-like cells (Figure 2A). Previous data have indicated that recurrent NPCOs are more easily generated than primary NPCOs. To determine the difference between primary and recurrent NPCOs, we analyzed the expression levels of stem cell markers. Our results showed that the percentage of BMI-1-expressing cells was higher (>80%) in the recurrent NPCOs than in the primary NPCOs (Figure 2B). We speculated that a higher expression of BMI-1 might aid in the generation of NPCOs, leading to the higher success rate of recurrent versus primary NPCO generation.
Challenges and expectations for the establishment of a recurrent NPCO biobank
We attempted to cryopreserve 39 NPCOs in liquid nitrogen for 6 months. Of these, 32 organoids were successfully revived. The NPCOs maintained good cell viability after long-term cryopreservation (Figure 3A). These 32 NPCOs were passaged for four generations while maintaining NPC cell viability. All the NPCOs had good cell viability within two generations (Figure 3A). After 4 generations, some of the NPCOs, like NPCO-P42 and NPCO-P52, grew more slowly, became blurry, and disintegrated, and some NPCOs, including NPCO-P44, did not meet the experimental criteria due to the small number and diameter of the organoid spheroids. In some NPCOs, like NPCO-P48, fibroblasts were activated and became dominant, significantly inhibiting organoid growth in 1 instance (Figure 3A). The fibroblasts used the cytokines necessary for organoid growth and enclosed the whole organoid, restricting its growth (Figure 3A). Because of these issues, we determined that the continued cultivation of NPCOs is currently difficult but worth investigating in the future.
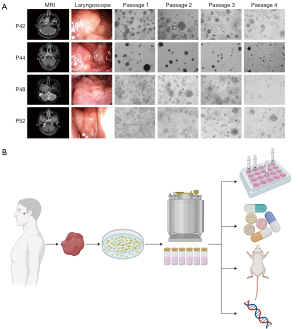
A biobank of recurrent NPC is still in its infancy. A tumor organoid biobank can recapitulate tumor heterogeneity and personalized genetic information (26-28), and a living biobank provides a rich resource and convenient platform for studying disease pathogenesis and enables the development of precision medicine (29). Here, we showed the potential workflow for establishing and applying a living biobank of recurrent NPCOs. The recurrent NPCOs could be deposited in a living biobank with detailed clinical information, then used for precision medicine, the development of new pharmaceuticals, experimental studies, and the construction of patient-derived organoid xenografts (PDOXs) for further basic and clinical research on recurrent or refractory NPC (Figure 3B).
Discussion
Immortalized cell lines are the most commonly used model in NPC research. Almost 100% of NPC cases are associated with EBV infection, but EBV genome were found lost in most of NPC cell lines (13,14). Additionally, several NPC cells have been found to be contaminated with HeLa cells. More and more scholars have raised questions: can cancer cell lines maintained the individual difference of the heterogeneity background and genetic characteristics after long-term in vitro culture and passage?
Patient-derived tumor xenografts (PDXs) have been used to establish tumors for NPC studies. But the low success rate and high cost limits the large-scale application of PDX model (30,31). Here, we established a patient-derived organoid model to overcome the drawbacks of existing NPC disease models. Nasopharyngeal carcinoma organoids can be used to screen out sensitive drugs for nasopharyngeal cancer cells in patients, guide clinicians to provide individualized drug regimens for patients with refractory nasopharyngeal carcinoma, improve the cure rate of patients, and prolong the life of patients. To provide reliable drug susceptibility data for the promotion of precise treatment of nasopharyngeal cancer.
Nasopharyngeal carcinoma tissue is usually obtained by endoscopic biopsy. Small tissue volume, low content of tumor cells and poor cell activity makes it a great challenge for in vitro culture of NPC organoid. In this study, we developed an optimal culture medium for NPCO. Matrigel is a reconstituted extracellular matrix containing metalloenzymes, cytokines, and laminin, which can promote cellular proliferation (32,33). Our result showed collagen II can also provide the scaffold function required for 3D growth of NPCO, but Matrigel mimic the in vivo tumor microenvironment during long-term culture. We also optimized the concentrations of Wnt3a, Rspo-1, EGF, Y27632, Noggin, and FGF2 in the NPCO culture medium. The Wnt/β-catenin signaling pathway is involved in NPC stem cell proliferation and self-renewal regulation in many tissues (13,34). Several studies have reported that Wnt3a can promote the differentiation of olfactory epithelium stem cells (32,33). EGF treatment activates EGFR signaling and promotes NPC cell proliferation and cell cycle progression (35). Similarly, FGF2 can promote stem cell division, proliferation, and apoptosis inhibition by activating the PI3K/PKB signaling pathway (36). Y27632 is known to be a selective inhibitor of the Rho-associated kinase p160-ROCK that reduces the loss of stem cells (21). The latest studies suggest that Y27632 promotes the establishment of NPC cell lines from NPC tissues (26,37). In this study, we generated NPCOs using our optimized NPCO culture medium.
We attempted to generate NPCOs using 62 NPC tissue samples. A total of 39 NPCOs were generated successfully. The success rates for primary and recurrent NPCOs were 47.06% and 81.25%, respectively. Almost 100% of NPC cases are associated with EBV infection, and we found that all our NPCOs carried EBV and maintained viral expansion. The NPCOs were mainly composed of cancer stem cells that exhibited rapid proliferation in the organoid culture medium. Additionally, stem cell markers were more highly expressed in the recurrent NPCOs than in the primary NPCOs. We believe that a higher expression of stem cell markers increases the success rate of the recurrent NPCOs.
However, there were some challenges to generating NPCOs, including the overexpansion of fibroblasts. NPC tissues have many infiltrated lymphocytes (20). To date, it has been difficult to maintain a coculture of lymphocytes and NPC stem cells to form a tumor microenvironment. It has been reported that T and B cell lymphocytes can secrete some cytokines to control the growth of fibroblasts (38,39). Schäffer et al. found that after several passages, immune cells stopped growing and gradually died off in the NPCOs and that concentrations of TGF-β and TNF-α decreased and could not inhibit the growth of fibroblasts, leading to the expansion of fibroblasts (40). Therefore, we believe that inhibiting the growth of fibroblasts is important for extending the passage numbers of NPCOs and that this is worth further investigation.
In conclusion, we successfully established a stable and efficient NPCO model using NPC tissues. All our NPCOs were EBV positive. We found that the NPCOs could retain the heterogeneity of parental tumors and recapitulate their pathophysiological characteristics. A biobank of NPCOs generated using tissues from patients with recurrent and refractory NPC has the potential to help us develop new anticancer agents, investigate the tumorigenesis mechanism, and develop individual therapies.
Acknowledgments
The authors wish to acknowledge Accurate International Biotechnology (Guangzhou) Co. Ltd., for the technical support of organoid culture and analysis.
Funding: This project was supported in part by the National Natural Science Foundation of China General Program (No. 81472534).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1076/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1076/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1076/coif). All authors report technical support from Accurate International Biotechnology (Guangzhou) Co. Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Biopsy and surgical resection samples were collected after written informed consent forms were obtained from the patients, and all related procedures were performed with the approval of the Research Ethics Committee at the Nanfang Hospital of the Southern Medical University (approval no. TCHIRB-10701103-E).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tian YM, Huang WZ, Lan YH, et al. Prognostic model and optimal treatment for patients with stage IVc nasopharyngeal carcinoma at diagnosis. Sci Rep 2019;9:19272. [Crossref] [PubMed]
- An F, Zhang Z, Xia M. Functional analysis of the nasopharyngeal carcinoma primary tumor-associated gene interaction network. Mol Med Rep 2015;12:4975-80. [Crossref] [PubMed]
- Xu FH, Xiong D, Xu YF, et al. An epidemiological and molecular study of the relationship between smoking, risk of nasopharyngeal carcinoma, and Epstein-Barr virus activation. J Natl Cancer Inst 2012;104:1396-410. [Crossref] [PubMed]
- Münz C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat Rev Microbiol 2019;17:691-700. [Crossref] [PubMed]
- Lee AWM, Lee VHF, Ng WT, et al. A systematic review and recommendations on the use of plasma EBV DNA for nasopharyngeal carcinoma. Eur J Cancer 2021;153:109-22. [Crossref] [PubMed]
- Zhou L, Chen J, Tao CJ, et al. Hematological Indexes Can Be Used to Predict the Incidence of Hypothyroidism in Nasopharyngeal Carcinoma Patients after Radiotherapy. Biomed Res Int 2020;2020:3860936. [Crossref] [PubMed]
- Si YF, Deng ZX, Weng JJ, et al. A study on the value of narrow-band imaging (NBI) for the general investigation of a high-risk population of nasopharyngeal carcinoma (NPC). World J Surg Oncol 2018;16:126. [Crossref] [PubMed]
- Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet 2019;394:64-80. [Crossref] [PubMed]
- Thamboo A, Patel VS, Hwang PH. 5-year outcomes of salvage endoscopic nasopharyngectomy for recurrent nasopharyngeal carcinoma. J Otolaryngol Head Neck Surg 2021;50:12. [Crossref] [PubMed]
- Chen W, Du M, Hu X, et al. Long noncoding RNA cytoskeleton regulator RNA promotes cell invasion and metastasis by titrating miR-613 to regulate ANXA2 in nasopharyngeal carcinoma. Cancer Med 2020;9:1209-19. [Crossref] [PubMed]
- Jin S, Li R, Chen MY, et al. Single-cell transcriptomic analysis defines the interplay between tumor cells, viral infection, and the microenvironment in nasopharyngeal carcinoma. Cell Res 2020;30:950-65. [Crossref] [PubMed]
- Liu Y, He S, Wang XL, et al. Tumour heterogeneity and intercellular networks of nasopharyngeal carcinoma at single cell resolution. Nat Commun 2021;12:741. [Crossref] [PubMed]
- Chan SY, Choy KW, Tsao SW, et al. Authentication of nasopharyngeal carcinoma tumor lines. Int J Cancer 2008;122:2169-71. [Crossref] [PubMed]
- Tsao SW, Tsang CM, To KF, et al. The role of Epstein-Barr virus in epithelial malignancies. J Pathol 2015;235:323-33. [Crossref] [PubMed]
- Cheung ST, Huang DP, Hui AB, et al. Nasopharyngeal carcinoma cell line (C666-1) consistently harbouring Epstein-Barr virus. Int J Cancer 1999;83:121-6. [Crossref] [PubMed]
- Bleijs M, van de Wetering M, Clevers H, et al. Xenograft and organoid model systems in cancer research. EMBO J 2019;38:e101654. [Crossref] [PubMed]
- Jee JH, Lee DH, Ko J, et al. Development of Collagen-Based 3D Matrix for Gastrointestinal Tract-Derived Organoid Culture. Stem Cells Int 2019;2019:8472712. [Crossref] [PubMed]
- Liu J, Li P, Wang L, et al. Cancer-Associated Fibroblasts Provide a Stromal Niche for Liver Cancer Organoids That Confers Trophic Effects and Therapy Resistance. Cell Mol Gastroenterol Hepatol 2021;11:407-31. [Crossref] [PubMed]
- Lucky SS, Law M, Lui MH, et al. Patient-Derived Nasopharyngeal Cancer Organoids for Disease Modeling and Radiation Dose Optimization. Front Oncol 2021;11:622244. [Crossref] [PubMed]
- Ding RB, Chen P, Rajendran BK, et al. Molecular landscape and subtype-specific therapeutic response of nasopharyngeal carcinoma revealed by integrative pharmacogenomics. Nat Commun 2021;12:3046. [Crossref] [PubMed]
- Xu H, Lyu X, Yi M, et al. Organoid technology and applications in cancer research. J Hematol Oncol 2018;11:116. [Crossref] [PubMed]
- Li X, Francies HE, Secrier M, et al. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat Commun 2018;9:2983. [Crossref] [PubMed]
- Tian Y, Ma X, Lv C, et al. Stress responsive miR-31 is a major modulator of mouse intestinal stem cells during regeneration and tumorigenesis. Elife 2017;6:29538. [Crossref] [PubMed]
- Broutier L, Mastrogiovanni G, Verstegen MM, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med 2017;23:1424-35. [Crossref] [PubMed]
- Fang H, Geng S, Hao M, et al. Simultaneous Zn2+ tracking in multiple organelles using super-resolution morphology-correlated organelle identification in living cells. Nat Commun 2021;12:109. [Crossref] [PubMed]
- Lin W, Yip YL, Jia L, et al. Establishment and characterization of new tumor xenografts and cancer cell lines from EBV-positive nasopharyngeal carcinoma. Nat Commun 2018;9:4663. [Crossref] [PubMed]
- Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol 2020;21:571-84. [Crossref] [PubMed]
- Lhousni S, Belmokhtar KY, Belmokhtar I, et al. Morocco's First Biobank: Establishment, Ethical Issues, Biomedical Research Opportunities, and Challenges. Biomed Res Int 2020;2020:8812609. [Crossref] [PubMed]
- Fan H, Demirci U, Chen P. Emerging organoid models: leaping forward in cancer research. J Hematol Oncol 2019;12:142. [Crossref] [PubMed]
- Vaes RDW, van Dijk DPJ, Welbers TTJ, et al. Generation and initial characterization of novel tumour organoid models to study human pancreatic cancer-induced cachexia. J Cachexia Sarcopenia Muscle 2020;11:1509-24. [Crossref] [PubMed]
- Costales-Carrera A, Fernández-Barral A, Bustamante-Madrid P, et al. Plocabulin Displays Strong Cytotoxic Activity in a Personalized Colon Cancer Patient-Derived 3D Organoid Assay. Mar Drugs 2019;17:648. [Crossref] [PubMed]
- Li M, Izpisua Belmonte JC. Organoids - Preclinical Models of Human Disease. N Engl J Med 2019;380:569-79. [Crossref] [PubMed]
- Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009;459:262-5. [Crossref] [PubMed]
- Dittmer DP, Hilscher CJ, Gulley ML, et al. Multiple pathways for Epstein-Barr virus episome loss from nasopharyngeal carcinoma. Int J Cancer 2008;123:2105-12. [Crossref] [PubMed]
- Liang J, Zheng S, Xiao X, et al. Epstein-Barr virus-encoded LMP2A stimulates migration of nasopharyngeal carcinoma cells via the EGFR/Ca2+/calpain/ITGβ4 axis. Biol Open 2017;6:914-22. [Crossref] [PubMed]
- Jiang H, Fan D, Zhou G, et al. Phosphatidylinositol 3-kinase inhibitor(LY294002) induces apoptosis of human nasopharyngeal carcinoma in vitro and in vivo. J Exp Clin Cancer Res 2010;29:34. [Crossref] [PubMed]
- Yip YL, Lin W, Deng W, et al. Establishment of a nasopharyngeal carcinoma cell line capable of undergoing lytic Epstein-Barr virus reactivation. Lab Invest 2018;98:1093-104. [Crossref] [PubMed]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214:199-210. [Crossref] [PubMed]
- Yu Q, Watson RR, Marchalonis JJ, et al. A role for T lymphocytes in mediating cardiac diastolic function. Am J Physiol Heart Circ Physiol 2005;289:H643-51. [Crossref] [PubMed]
- Schäffer M, Barbul A. Lymphocyte function in wound healing and following injury. Br J Surg 1998;85:444-60. [Crossref] [PubMed]
(English Language Editor: L. Roberts)


