Choice of crystalloids in sepsis: a conundrum waiting to be solved
One of the mainstays of sepsis and septic shock management is early intravenous fluid resuscitation to correct intravascular hypovolemia and restore adequate perfusion (1). There is an ongoing controversy on the optimal volume and choice of intravenous fluids to be administered (2,3). Recent systematic reviews and meta-analyses have concluded that pentastarch and hydroxyethyl starch are inferior to crystalloids as starch solutions increase the risk of kidney injury and death in patients with sepsis (4,5). Non-synthetic colloids such as albumin demonstrated no additional measurable harm or benefit when compared to crystalloids in sepsis (6,7). While the debate on crystalloids vs. non-synthetic colloids continues, another deliberation regarding the choice of crystalloids; ‘balanced’ vs. ‘non-balanced’, has started to garner interest.
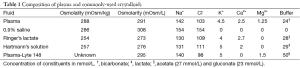
Saline (0.9% NaCl solution), also widely known as ‘normal’ saline or ‘physiological’ saline is by far the most commonly used intravenous solution in the world with over 200 million liters sold annually in the United States alone (8). Over 1 million liters of intravenous saline are administered to patients worldwide daily (9). Paradoxically, normal saline has been identified as neither normal nor physiological in an editorial way back in 1970 (10). It is considered ‘non-balanced’ due to its supra-physiological concentration of chloride ions (Table 1). Liberal administration of saline has been shown to result in hyperchloremia and metabolic acidosis (11,12). Hyperchloremia is postulated to mediate vascular smooth muscle contraction, which potentiates norepinephrine and angiotensin II-induced vasoconstriction thus reducing renal blood flood via tubulo-glomerular feedback (13,14). In addition, excessive salt administration causes decreased diuresis, fluid overload and interstitial edema leading to further reduction in renal blood flow (15). Both mechanisms exacerbate the risk of pre-renal kidney injury. The hyperchloremic metabolic acidosis may also have deleterious effects on the immune system that is demonstrated by increased plasma nitric oxide levels and pro-inflammatory cytokines (16).
The search for the ideal fluid for resuscitative use, which should best resemble constituents of human plasma, has led to the development of ‘balanced’ solutions that have minimal effect on the acid-base equilibrium, or with a physiological or low content of chloride. Examples of such solutions include Ringer’s lactate, Hartmann’s solution, Plama-Lyte and Sterofundin (Table 1). Many have jumped on the recent bandwagon to reduce the use of chloride-rich solutions in favour of balanced solutions. The current clinical evidence for the use of balanced solutions, particularly in sepsis and septic shock is largely drawn from observational studies involving patients with sepsis, septic shock or systemic inflammatory response syndrome (17,18). Other observational studies from intensive care units have also demonstrated decreased risk of acute kidney injury in patients receiving balanced solutions compared to saline (19-21). However, mixtures of intravenous fluids are frequently used in clinical practice, and it is unclear if morbidity and mortality are influenced by different mixtures of fluids.
A study by Raghunathan and colleagues published in 2015 sought to test the hypothesis that specific mixtures of intravenous fluids during initial resuscitation in patients with sepsis are associated with outcomes such as mortality, length of hospital stay and cost (22). This retrospective cohort study included 60,734 patients with sepsis over 5 years (from 2006 to 2010) from 360 intensive care units across the United States. Four mutually exclusive categories were compared with one another: (I) patients who received saline exclusively; (II) patients who received saline and balanced crystalloid solutions; (III) patients who received saline and colloids (either hydroxyethyl starch or albumin); and (IV) patients who received all three types of fluids. After inverse probability weighting-based adjustment, patients who received saline and balanced solutions had the lowest in-hospital mortality of 17.7%. The effects were maintained even after hierarchical logistic regression modelling and pairwise propensity score matching on day 2 of hospitalization. They also showed that treatment with colloids resulted in increased mortality when balanced crystalloids were not coadministered and no difference in survival when balanced crystalloids were coadministered. Therefore, the authors surmised that the distinction between types of crystalloids used were more significant than the crystalloid vs. colloid differentiation.
The results of this study by Raghunathan and colleagues seem to back up previous systematic reviews on the deleterious effects specifically of synthetic colloids from previous systematic reviews and meta-analyses (4,5). The main drawback of this study is its retrospective nature, though the authors have commendably gone through great lengths using statistical methods to control for confounding. Another limitation is the use of administrative and financial data rather than actual chart reviews. Furthermore, only 9.2% of those meeting the inclusion criteria were finally analysed after various exclusion criteria were applied and only included vasopressor-dependent sepsis, further limiting its generalizability (22). Nevertheless, this study is currently the only one that tried to examine pragmatically how real-world use of mixtures of fluids is associated with clinically important outcomes. It is likely that the practice of using different solutions at different times is prevalent worldwide. Despite its shortcomings, the results of this study may shed some light into the effects of various combinations of intravenous fluids in critically ill patients.
The benefits of balanced solutions have also been demonstrated in other clinical scenarios where the patients required large amounts of intravenous fluids. In perioperative care, the administration of balanced solutions to adult and pediatric patients in surgery was shown to be associated with less metabolic derangement, in particular hyperchloremia and metabolic acidosis (23). Similar associations were also demonstrated in patients who suffered acute severe traumatic injuries requiring fluid and blood transfusion (24). In patients requiring major open abdominal surgery, treatment with balanced solutions was associated with fewer complications, namely postoperative infection, renal failure requiring dialysis, blood transfusion, electrolyte disturbance, acidosis investigation and intervention (8).
The maelstrom concerning the use of saline mainly centers on its chloride content. The possibility of hypernatremia and its association with adverse outcomes has not been addressed in detail (25). Of note are the differences in osmolarity and osmolality between saline and balanced solutions (Table 1) (26). The values of osmolarity and osmolality are interchangeable in dilute physiological solutions. However, incomplete ionization of the solutes in balanced solutions like Ringer’s lactate and Hartmann’s solution renders them hypotonic compared to normal plasma in vivo (27). A study on human volunteers showed that infusion of large volumes of Ringer’s lactate decreased serum osmolality and shorter time to first urine output (28). It was postulated that the inhibition of release of antidiuretic hormone resulted in this finding. Such disparity needs to be considered from a mechanistic perspective in future studies.
Full table
While the presence of hyperchloremic acidosis is irrefutable in saline infusion, the degree of adverse effects is directly related to the amount of fluid administered (29). Correction of hyperchloremic acidosis alone is unlikely to lead to substantial clinical benefits as it has been considered inconsequential, resolving within a day if appropriate amounts of saline are administered (30). The lack of potassium in saline solution may be viewed as an advantage in some conditions such as renal failure where risk of hyperkalemia is relatively higher. The use saline infusion in other conditions such as diabetic ketoacidosis and traumatic brain injury is currently still a subject to considerable disagreement.
The only randomized trial done thus far to compare saline vs. balanced solutions in intensive care units was recently published (31). Plasma-Lyte 148 was compared to saline in a multi-center, cluster-randomized, double-crossover study that failed to demonstrate any difference in risk of acute kidney injury [relative risk (RR), 1.04; 95% confidence interval (CI), 0.80–1.36], requirements of renal replacement therapy (RR, 0.96; 95% CI, 0.62–1.50) and mortality (RR, 0.88; 95% CI, 0.67–1.17) at 90 days in 2,092 patients in the intensive care unit. Although the trial is of a superior design compared to previous observational studies, the study population consisted of mainly non-septic surgical patients who had a low overall incidence of acute kidney injury (9.4%) and mortality (8.0%). The very small subgroup analysis of patients with sepsis (n=77) demonstrated a higher incidence of acute kidney injury (20.8%) and mortality (15.5%). Thus, the treatment effect of balanced solutions in this low-risk group may be underestimated.
In conclusion, based on current, predominantly observational evidence, it is justifiable to consider balanced solutions as the first choice crystalloids for resuscitation of septic patients. The solution (pun intended) to the conundrum of which is the ideal crystalloid to use in sepsis is far from close. Further multicenter randomized trials including medium to high risk septic patients are required to arrive at more robust conclusions and provide more concrete recommendations.
Acknowledgements
None.
Footnote
Provenance: This is a Guest commentary commissioned by Guest Editor Zhongheng Zhang, MD (Department of Critical Care Medicine, Jinhua Municipal Central Hospital, Jinhua Hospital of Zhejiang University, Jinhua, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637. [Crossref] [PubMed]
- Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med 2013;369:1243-51. [Crossref] [PubMed]
- Finfer S. Clinical controversies in the management of critically ill patients with severe sepsis: resuscitation fluids and glucose control. Virulence 2014;5:200-5. [Crossref] [PubMed]
- Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA 2013;309:678-88. [Crossref] [PubMed]
- Rochwerg B, Alhazzani W, Sindi A, et al. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med 2014;161:347-55. [Crossref] [PubMed]
- Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004;350:2247-56. [Crossref] [PubMed]
- Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med 2014;370:1412-21. [Crossref] [PubMed]
- Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg 2012;255:821-9. [Crossref] [PubMed]
- Awad S, Allison SP, Lobo DN. The history of 0.9% saline. Clin Nutr 2008;27:179-88. [Crossref] [PubMed]
- Wakim KG. "Normal" 0.9 per cent salt solution is neither "normal" nor physiological. JAMA 1970;214:1710. [Crossref] [PubMed]
- Scheingraber S, Rehm M, Sehmisch C, et al. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology 1999;90:1265-70. [Crossref] [PubMed]
- McFarlane C, Lee A. A comparison of Plasmalyte 148 and 0.9% saline for intra-operative fluid replacement. Anaesthesia 1994;49:779-81. [Crossref] [PubMed]
- Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest 1983;71:726-35. [Crossref] [PubMed]
- Chowdhury AH, Cox EF, Francis ST, et al. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg 2012;256:18-24. [Crossref] [PubMed]
- Mårtensson J, Bellomo R. Are all fluids bad for the kidney? Curr Opin Crit Care 2015;21:292-301. [Crossref] [PubMed]
- Zhou F, Peng ZY, Bishop JV, et al. Effects of fluid resuscitation with 0.9% saline versus a balanced electrolyte solution on acute kidney injury in a rat model of sepsis*. Crit Care Med 2014;42:e270-8. [Crossref] [PubMed]
- Raghunathan K, Shaw A, Nathanson B, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis*. Crit Care Med 2014;42:1585-91. [Crossref] [PubMed]
- Shaw AD, Raghunathan K, Peyerl FW, et al. Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive Care Med 2014;40:1897-905. [Crossref] [PubMed]
- Yunos NM, Bellomo R, Hegarty C, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 2012;308:1566-72. [Crossref] [PubMed]
- Yunos NM, Bellomo R, Glassford N, et al. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intensive Care Med 2015;41:257-64. [Crossref] [PubMed]
- Krajewski ML, Raghunathan K, Paluszkiewicz SM, et al. Meta-analysis of high-versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg 2015;102:24-36. [Crossref] [PubMed]
- Raghunathan K, Bonavia A, Nathanson BH, et al. Association between initial fluid choice and subsequent in-hospital mortality during the resuscitation of adults with septic shock. Anesthesiology 2015;123:1385-93. [Crossref] [PubMed]
- Disma N, Mameli L, Pistorio A, et al. A novel balanced isotonic sodium solution vs normal saline during major surgery in children up to 36 months: a multicenter RCT. Paediatr Anaesth 2014;24:980-6. [Crossref] [PubMed]
- Young JB, Utter GH, Schermer CR, et al. Saline versus Plasma-Lyte A in initial resuscitation of trauma patients: a randomized trial. Ann Surg 2014;259:255-62. [Crossref] [PubMed]
- O'Donoghue SD, Dulhunty JM, Bandeshe HK, et al. Acquired hypernatraemia is an independent predictor of mortality in critically ill patients. Anaesthesia 2009;64:514-20. [Crossref] [PubMed]
- Severs D, Hoorn EJ, Rookmaaker MB. A critical appraisal of intravenous fluids: from the physiological basis to clinical evidence. Nephrol Dial Transplant 2015;30:178-87. [Crossref] [PubMed]
- Guidet B, Soni N, Della Rocca G, et al. A balanced view of balanced solutions. Crit Care 2010;14:325. [Crossref] [PubMed]
- Williams EL, Hildebrand KL, McCormick SA, et al. The effect of intravenous lactated Ringer's solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg 1999;88:999-1003. [PubMed]
- Ince C, Groeneveld AB. The case for 0.9% NaCl: is the undefendable, defensible? Kidney Int 2014;86:1087-95. [Crossref] [PubMed]
- Guidet B, Martinet O, Boulain T, et al. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care 2012;16:R94. [Crossref] [PubMed]
- Young P, Bailey M, Beasley R, et al. Effect of a Buffered Crystalloid Solution vs Saline on Acute Kidney Injury Among Patients in the Intensive Care Unit: The SPLIT Randomized Clinical Trial. JAMA 2015;314:1701-10. [Crossref] [PubMed]


