
Risk factors for C5 palsy following the posterior spinal process-splitting laminoplasty for cervical ossification of the posterior longitudinal ligament: a case control study
Introduction
C5 palsy is a common complication after posterior cervical laminoplasty, which can directly affect the immediate outcome and patient satisfaction (1,2). Even with identical surgical approaches, the incidence of C5 palsy varies by disease type. Wu et al. (3) found the incidence of C5 palsy in patients with cervical ossification of the posterior longitudinal ligament (C-OPLL) was 9.2 times that in patients with cervical spondylolisthesis, and proposed that C-OPLL may be a risk factor for C5 palsy after posterior cervical decompression. Shou et al. (4) reported similar findings in their meta-analysis; the incidence of C5 palsy after surgery for C-OPLL was 5.8%, which was higher than that in patients with cervical spondylosis (4.5%). In another study, the mean incidence of C5 palsy following posterior cervical surgery for C-OPLL was 8.3% (range, 3.2–28.6%) (5), while Wang et al. (6) reported that the incidence of C5 palsy even reached 25% after a posterior French-door laminoplasty in C-OPLL patients with a spinal canal occupancy ratio of >50%.
Not only the variety of its incidence, the exact etiology of C5 palsy remains unclear. The possible common risk factors include C4/5 foraminal stenosis, iatrogenic injuries, embolic effects, and spinal cord gray matter degeneration (T2 high-signal shadow) (7), but a consensus has yet to be reached. Posterior spinal process-splitting laminoplasty (pSPSL), which belongs to the modified posterior French-door laminoplasty, has long been performed in our center for the treatment of C-OPLL. Herein, we retrospectively analyzed incidence and risk factors of C5 palsy after this procedure, in order to find the potential pathomechanism of this complication. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1730/rc).
Methods
General data
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Beijing Jishuitan Hospital (No. 202201-31). Informed consent was taken from all the patients. The clinical and imaging data of 220 patients who underwent pSPSL for C-OPLL at our center from January 2018 to December 2019 were retrospectively collected. The inclusion criteria were as follows: (I) patients younger than 80 years; (II) those with computed tomography (CT)-confirmed C-OPLL; and (III) patients with functional involvement of the spinal cord but without motor abnormalities in the area innervated by the C5 nerve roots. The exclusion criteria were as follows: (I) patients with a previous history of cervical spine surgery; (II) patients that had undergone combined anterior/posterior surgery; (III) those receiving revision surgery in situ during follow-up; (IV) patients with a history of postoperative infection; and (V) patients who failed to complete the follow-up for any reason. The diagnostic criteria for C5 palsy were as follows: (I) unilateral or bilateral deltoid muscle strength [evaluated by the Manual Muscle Testing (MMT) scale] ≤ grade 3 within 2 weeks after surgery; (II) with or without biceps muscle involvement; and (III) without signs of further deterioration of spinal cord function (8).
Surgical method
Surgical incision and approach
A C2–7 posterior median cervical incision was made, in which the skin and subcutaneous tissue were cut open, and the cervical ligaments and soft tissues of the neck were divided layer-by-layer with a high-intensity focused ultrasound (HIFU) to expose the C3–6 spinous processes and the superior margin of the C7 spinous process. The paravertebral muscles were pushed away from the periosteum on the cervical spinous process using a Cobbs periosteal stripper to expose the C3 to C6 vertebral laminae and the upper half of the C7 vertebral lamina. Caution was taken to preserve the semispinalis cervicis at the insertion of the C2 spinous process.
Spinous process-splitting and gutter preparation
The C3 lamina was excised using an abrasion drill, curette, and a 2-mm Kerrison rongeur. If the OPLL site extended upwards and exceeded the height of the C3 vertebral body, dome decompression at the inferior portion of the C2 lamina was performed. The ligamentum flavum between C6 and C7 was removed with the Kerrison rongeur and forceps and the upper part of the C7 lamina was excised using the abrasion drill and the Kerrison rongeur. Hollow plastic catheters were placed under the C4 to C6 laminae, and a T-saw (titanium wire saw) was inserted through the catheters to enable the symmetrical sawing of the C4–6 spinous processes. Two gutters were ground along the junction between the facet joints and the vertebral laminae on both sides of C4 to C6 using the abrasion drill. These gutters were about 3.5 mm in width and extended deeply into the contralateral cortical bones. The splitted vertebral laminae were slowly separated on both sides, with the gutters as hinges, to expose the underlying dura.
Implantation of artificial bone spacers
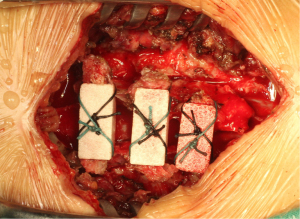
A 1-mm drill was used to punch a hole in the center of the splitted spinal process on both sides. Three trapezoidal coral artificial bones (approximately 2.6 cm3 in size) were sequentially passed through the prefabricated orifice of the spinal process using two 10-gauge sutures, and then cross-fixed to the split spinous processes between C4 and C6 (Figure 1).
Wound closure
The paravertebral muscles, cervical ligaments, subcutaneous tissue, and skin were sutured layer-by-layer. A negative-pressure drainage was placed at the surgical area.
Imaging evaluation
The imaging modalities included cervical spine X-ray and CT before and after surgery, as well as magnetic resonance imaging (MRI) before surgery. Preoperative cervical neutral upright radiographs were used to assess the K-line (i.e., the line between the midpoints of the spinal canal at C2 and C7 on standard lateral cervical radiographs). If the apex of the OPLL foci did not exceed this line, the K-line was considered positive, and vice versa (9). Preoperative CT was used for typing the C-OPLL. A diagnosis of foraminal stenosis was made if the width of the C4/5 intervertebral foramen was <2 mm (at the narrowest level) on preoperative axial CT. On a postoperative cross-sectional CT scan, the gutters should be located at the junction between the vertebral laminae and the medial edge of the facet joint. If the lateral edge of the gutter was located on the laminae and did not reach the facet joint, the gutter was positioned medially, which was considered to be gutter malposition. If high signals of the spinal cord were present at the C4/5 on preoperative T2-weighted imaging, it was considered a positive preoperative spinal cord signal change. To reduce data bias, the imaging parameters were averaged after measurement by one clinician and one radiologist who did not participate in the procedure.
Statistical analysis
Age was described as mean ± standard deviation, while sex, OPLL type, K-line, foraminal stenosis, gutter malposition, and preoperative spinal cord signal change were presented using frequencies (percentages). The comparisons between sex, OPLL type, K-line, foraminal stenosis, gutter malposition, and preoperative spinal cord signal change between the postoperative palsy group (group A) and non-palsy group (group B) were based on the chi-square test. For parameters that did not meet the conditions of the chi-square test, Fisher’s exact test was adopted for P value estimation. Logistic regression was used to analyze the independent risk factors for postoperative palsy (Table 1). All statistical analyses were performed using SPSS 21.0 software package, the single significant factor and the clinically significant factor were included in the logistic regression model, the Wald chi-square value was calculated by Wald test, and the factor with P value less than 0.05 was identified as an independent risk factor, the test level was 0.05 and it was bilateral.
Table 1
| Variables | Assignment |
|---|---|
| Postoperative palsy | 0: no; 1: yes |
| OPLL type | 0: segmental type; 1: continuous type; 2: mixed type |
| K-line | 0: negative; 1: positive |
| Gutter malposition | 0: negative; 1: positive |
| Foraminal stenosis | 0: negative; 1: positive |
| Preoperative spinal cord signal change | 0: negative; 1: positive |
OPLL, ossification of posterior longitudinal ligament.
Results
General data
After excluding nine patients due to incomplete follow-up information, 211 eligible cases were eventually included in the study. All surgical procedures were performed by senior spinal surgeons with more than 8 years of experience in this specialty, and there were no differences in the surgical techniques applied. There were 193 patients in group B (without C5 palsy) and 18 patients in group A (with C5 palsy), and the incidence of C5 palsy was 8.53%. C5 palsy occurred in none of the patients within 1 day, three patients within 2–3 days, and 15 patients within 4–7 days after surgery. The MMT grade decreased from 5 before surgery to 3 after surgery in 16 patients and from 5 to 2 in two patients. During the follow-up (mean duration: 25.10±6.67 months), the MMT grade increased from 3 to 5 in 16 patients and from 2 to 4 in one patient. In another patient, the MMT grade recovered from 2 to 3.
Statistical results
There were no statistically significant differences between groups A and B in terms of age, gender, OPLL type, K-line, and positive preoperative spinal cord signal change (i.e., high signals on T2-weighted imaging). The proportion of patients with foraminal stenosis was 72.2% (13/18) in group A and 19.7% (38/193) in group B (P<0.001). The proportion of patients with gutter malposition was 72.2% (13/18) in group A and 15.5% (30/193) in group B (P<0.001) (Table 2).
Table 2
| Factors | Group A (n=18) | Group B (n=193) | P value |
|---|---|---|---|
| Age (years) | 61.7±9.0 | 59.0±7.7 | 0.172 |
| Gender | |||
| Male | 10 (55.6) | 102 (52.8) | 0.826 |
| Female | 8 (44.4) | 91 (47.2) | |
| OPLL type | 0.963 | ||
| Segmental type | 3 (16.7) | 34 (17.6) | |
| Continuous type | 6 (33.3) | 69 (35.8) | |
| Mixed type | 9 (50.0) | 90 (46.6) | |
| K-line (+) | 15 (83.3) | 148 (76.7) | 0.520 |
| Foraminal stenosis (+) | 13 (72.2) | 38 (19.7) | <0.001* |
| Gutter malposition (+) | 13 (72.2) | 30 (15.5) | <0.001* |
| Preoperative spinal cord signal change (+) | 5 (27.8) | 50 (25.9) | <0.787* |
Data are shown as mean ± SD or n (%). *, Fisher exact test. +, positive. OPLL, ossification of posterior longitudinal ligament.
Factors that differed between groups A and B (i.e., foraminal stenosis and gutter malposition) and other factors (including OPLL type, K-line, and preoperative spinal cord signal change) were included for regression analysis, and the results showed that gutter malposition and foraminal stenosis were independent risk factors for C5 palsy (P<0.001). The risk of developing C5 palsy was 11.073 times higher in patients with gutter malposition than in those without, and 8.455 times higher in patients with foraminal stenosis than in those without. Compared to patients with segmental OPLL, the risk of developing C5 palsy was 1.357 times higher in patients with continuous OPLL and 8.455 times higher in patients with mixed OPLL; however, these differences were not statistically significant. Furthermore, the risk of developing C5 palsy was 1.492 times higher in patients with positive K-line and 1.251 times higher in patients with positive preoperative spinal cord signal change, although these differences were also not statistically significant (Table 3).
Table 3
| Variables | Regression coefficient | Standard error | Wald value | P value | OR (95% CI) |
|---|---|---|---|---|---|
| OPLL type | |||||
| Segmental type | – | – | – | – | 1.000 |
| Continuous type | 0.305 | 0.867 | 0.124 | 0.725 | 1.357 (0,248, 7.421) |
| Mixed type | 0.456 | 0.821 | 0.308 | 0.579 | 1.577 (0,316, 7.880) |
| K-line (+) | 0.400 | 0.771 | 0.269 | 0.604 | 1.492 (0.329, 6.757) |
| Gutter malposition (+) | 2.404 | 0.601 | 16.016 | <0.001 | 11.073 (3.411, 35.948) |
| Foraminal stenosis (+) | 2.135 | 0.610 | 12.256 | <0.001 | 8.455 (2.559, 27.936) |
| Preoperative spinal cord signal change (+) | 0.224 | 0.665 | 0.113 | 0.737 | 1.251 (0.340, 4.602) |
| Constant | −5.046 | 1.159 | 18.968 | <0.001 | 0.006 |
+, positive. OPLL, ossification of posterior longitudinal ligament; OR, odds ratio; CI, confidence interval.
Discussion
For patients undergoing pSPSL for C-OPLL in our center, the incidence of C5 palsy was 8.53%, which was congruous to that previously reported in the literature (8.3%) (5). We found that C4/5 foraminal stenosis and gutter malposition were risk factors for C5 palsy after surgery. The prognoses of the cases analyzed in this study were good. Deltoid muscle strength was recovered to MMT grade 5 in 16 patients at the last follow-up visits; meanwhile, muscle strength in the remaining two patients with postoperative muscle strength ≤ grade 2 eventually increased to grade 4 in one case and to grade 3 in the other case. Modified posterior French-door laminoplasty was performed in our center. Sakaura et al. (5) and Park et al. (10) reported that the incidence of C5 palsy was lower after a French-door procedure than after an open-door procedure. Park et al. (10) also argued that proper gutter preparations inside the facet joints bilaterally was effective in preventing C5 palsy. In their meta-analysis, Luo et al. (11) also suggested that the incidence of C5 palsy was higher after a open-door surgery than after a French-door surgery, although the difference was not significant. Takemitsu et al. (12) reported that the risk of C5 palsy after posterior fusion surgery was 11.6 times higher than that after non-fusion surgery. Therefore, despite the incidence of C5 palsy, patients undergoing pSPSL in our center had an excellent overall prognosis, and pSPSL is a feasible posterior decompression modality for C-OPLL patients.
Numerous studies have suggested that preoperative C4/5 foraminal stenosis is associated with the development of C5 palsy after a cervical spine surgery (3,13,14). Cross-sectional CT measurement of the foramina at the C4/5 levels revealed that the incidence of C5 palsy was significantly higher when the transverse diameter was <2 mm, mainly due to the posterior shift of the spinal cord after laminoplasty, which increased the strain on the C5 nerve root at the C4/5 foraminal stenosis and induced C5 palsy (14). Thus, the diagnostic criterion for C4/5 foraminal stenosis in this study was a transverse diameter of <2 mm on CT images. Katsumi et al. (15) demonstrated that there was a statistically significant difference in foraminal diameter between the C5 palsy and non-palsy groups. Takeuchi et al. (16) also reported that C4/5 foraminal stenosis resulted in an increase in internal pressure in the C5 nerve root due to poor circulation, eventually leading to swelling, which manifested as an increase in the cross-sectional area of the C5 nerve root on ultrasonography. This made it more susceptible to palsy symptoms due to spinal cord traction. Sasai et al. (17) proposed that preoperative subclinical compression of the C5 nerve root by the C4/5 intervertebral foramen is a possible pathogenic mechanism of postoperative C5 palsy. In their study, preoperative electromyographic testing identified 33 clinical C5 nerve root lesions in 23 patients. C5 palsy was avoided by prophylactic micro-foraminotomy. In addition, Imagama et al. (18) argued that foraminotomy should be reasonably performed and excessive removal of the lamina is not advisable. For patients who develop C5 palsy only after surgery, remedial foraminotomy can be performed if necessary, which can also offer satisfactory results. In particular, for patients who develop severe C5 palsy postoperatively (MMT grade 0 or 1), early C4/5 foraminotomy may be performed to help speed up recovery (19). However, Katsumi et al. (20) found that although prophylactic foraminotomy significantly reduced the incidence of the aforementioned complications, a small number of patients still developed C5 palsy postoperatively. Thus, the assumption that foraminal stenosis may cause C5 palsy needs to be further investigated.
In the present study, gutter malposition during decompression was found to be a risk factor for postoperative C5 palsy. Gutter malposition refers to the gutter being too far inwards and located on the vertebral laminae rather than at the transitional zone between the vertebral laminae and the facet joint. Gutter malposition increases the risk of C5 palsy in two ways: (I) since the thickness of the vertebral laminae is notably lower than that of the transitional zone between the vertebral laminae and the facet joint, the inner cortical bone of the vertebral laminae can be easily breached during drilling of the gutter, leading to direct injury or thermal burn to the nerve, as demonstrated in previous reports (21,22); and (II) it aggravates the excessive traction of the nerve root cuff: after the spinal canal is enlarged and re-shaped intraoperatively, the spinal cord drifts posteriorly together with the proximal end of the nerve root cuff, while the distal end of the nerve root cuff is bound between the residual laminae and OPLL foci due to the inward position of the gutter, with limited movability. This causes traction of the portion of the nerve root sleeve within the dura, which eventually leads to the symptoms of C5 palsy (a typical case is shown in Figure 2A-2D). This is one of the features that distinguishes C-OPLL from cervical spinal stenosis and cervical disc herniation, both of which are dominated by soft intervertebral discs or osteophytes that cause compression, rather than forming hard compression as in the case of C-OPLL foci. Kurosa et al. (23) also found that for patients with C-OPLL, their C5 nerve root sleeves can adhere to the surrounding ligamentous ossification foci, triggering postoperative nerve root palsy. If the gutter is positioned medially, the residual laminae will compress the distal end of the nerve root sleeve at the ossification foci dorsal-ventrally, limiting its movement and causing it to be pulled dorsally from the proximal end, thereby triggering palsy. However, in open-door laminoplasty, Nakajima et al. (8) found that if the gutter was outwardly positioned, the C5 nerve root would be further stretched at the narrowed foramen, leading to C5 palsy, although this only occurred in patients with C4/5 foraminal stenosis. Nevertheless, unlike foraminal stenosis, the outward position of the gutter is not considered an independent risk factor for C5 palsy. In the present study, the posterior shift of the spinal cord was somewhat limited due to the inward position of the gutter, which may be beneficial in preventing C5 palsy. According to previous studies, the mean distance of spinal cord posterior shift is 5–5.5 mm in patients with C5 palsy, compared with only 2–3.3 mm in non-palsy patients, which is a statistically significant difference (24,25). However, some recent studies also revealed that the degree of spinal cord posterior shift does not significantly correlate with C5 palsy (3,26). Thus, compared with spinal cord posterior shift, gutter malposition has a greater impact on the occurrence of C5 palsy.

K-line negativity often suggests cervical kyphosis and/or ossification foci with a high canal occupancy ratio in C-OPLL patients. In K-line (−) patients with adequate cervical lordosis, there is a risk of developing distraction spinal cord injury and even neurological impairment after a posterior cervical spine surgery (9). There is likewise an increased risk of C5 palsy due to nerve root stretching (27). However, we did not find that K-line (−) was a risk factor for C5 palsy, probably because the French-door procedure uses a trapezoidal “artificial bone” as a spacer, which differs from that in an open-door procedure or laminectomy. The degree of “door-opening” is controllable, and thus can prevent the traction lesions of nerves due to excessive posterior shift of the spinal cord. In addition, possible mechanisms for C5 palsy may also include intraoperative nerve injury, the opening angle of an open-door procedure, changes in cervical curvature, altered spinal cord signals, and ischemia-reperfusion injury to the spinal cord (28,29), although none of these have been fully confirmed.
Our current study had some limitations that should be noted. Firstly, the sample size was small due to its retrospective single-center design. Also, routine postoperative MRI was not performed in all cases, so the extent of spinal cord posterior shift could not be evaluated. MRI is valuable in assessing the internal morphology of spinal cord; however, postoperative MRI was omitted in our study because none of our patients showed progressive deterioration of spinal cord function shortly after surgery and the prognosis for C5 palsy is typically good. Nonetheless, the risk factors for C5 palsy were identified in our current study, which may facilitate effective prevention of this complication in the future.
Although the incidence of C5 palsy reached 8.53% after pSPSL for C-OPLL, the prognosis of patients is good, and muscle strength recovered well in most patients. C4/5 foraminal stenosis and gutter malposition were identified as the risk factors for C5 palsy.
Acknowledgments
Funding: This study was supported by the Beijing JST Research Horizontal Subject (No. 2-3-1-1-164-02).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1730/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1730/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1730/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Beijing Jishuitan Hospital (No. 202201-31). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kong QJ, Luo X, Tan Y, et al. Anterior Controllable Antedisplacement and Fusion (ACAF) vs Posterior Laminoplasty for Multilevel Severe Cervical Ossification of the Posterior Longitudinal Ligament: Retrospective Study Based on a Two-Year Follow-up. Orthop Surg 2021;13:474-83. [Crossref] [PubMed]
- Lee JJ, Kim HC, Jeon HS, et al. Laminectomy with instrumented fusion vs. laminoplasty in the surgical treatment of cervical ossification of the posterior longitudinal ligament: A multicenter retrospective study. J Clin Neurosci 2021;94:271-80. [Crossref] [PubMed]
- Wu FL, Sun Y, Pan SF, et al. Risk factors associated with upper extremity palsy after expansive open-door laminoplasty for cervical myelopathy. Spine J 2014;14:909-15. [Crossref] [PubMed]
- Shou F, Li Z, Wang H, et al. Prevalence of C5 nerve root palsy after cervical decompressive surgery: a meta-analysis. Eur Spine J 2015;24:2724-34. [Crossref] [PubMed]
- Sakaura H, Hosono N, Mukai Y, et al. C5 palsy after decompression surgery for cervical myelopathy: review of the literature. Spine (Phila Pa 1976) 2003;28:2447-51. [Crossref] [PubMed]
- Wang H, Chen J, Ma YX, et al. Correlation between vertebral canal occupying ratio of severe cervical ossification of posterior longitudinal ligament and postoperative C5 palsy. Journal of Spinal Surgery 2018;16:31-4.
- Wang T, Wang H, Liu S, et al. Incidence of C5 nerve root palsy after cervical surgery: A meta-analysis for last decade. Medicine (Baltimore) 2017;96:e8560. [Crossref] [PubMed]
- Nakajima H, Kuroda H, Watanabe S, et al. Risk factors and preventive measures for C5 palsy after cervical open-door laminoplasty. J Neurosurg Spine 2019; Epub ahead of print. [Crossref] [PubMed]
- Fujiyoshi T, Yamazaki M, Kawabe J, et al. A new concept for making decisions regarding the surgical approach for cervical ossification of the posterior longitudinal ligament: the K-line. Spine (Phila Pa 1976) 2008;33:E990-3. [Crossref] [PubMed]
- Park JH, Roh SW, Rhim SC, et al. Long-term outcomes of 2 cervical laminoplasty methods: midline splitting versus unilateral single door. J Spinal Disord Tech 2012;25:E224-9. [Crossref] [PubMed]
- Luo W, Li Y, Zhao J, et al. Open-versus French-Door Laminoplasty for the Treatment of Cervical Multilevel Compressive Myelopathy: A Meta-Analysis. World Neurosurg 2018;117:129-36. [Crossref] [PubMed]
- Takemitsu M, Cheung KM, Wong YW, et al. C5 nerve root palsy after cervical laminoplasty and posterior fusion with instrumentation. J Spinal Disord Tech 2008;21:267-72. [Crossref] [PubMed]
- Gu Y, Cao P, Gao R, et al. Incidence and risk factors of C5 palsy following posterior cervical decompression: a systematic review. PLoS One 2014;9:e101933. [Crossref] [PubMed]
- Lee HJ, Ahn JS, Shin B, et al. C4/5 foraminal stenosis predicts C5 palsy after expansive open-door laminoplasty. Eur Spine J 2017;26:2340-7. [Crossref] [PubMed]
- Katsumi K, Yamazaki A, Watanabe K, et al. Analysis of C5 palsy after cervical open-door laminoplasty: relationship between C5 palsy and foraminal stenosis. J Spinal Disord Tech 2013;26:177-82. [Crossref] [PubMed]
- Takeuchi M, Wakao N, Kamiya M, et al. Simple presurgical method of predicting C5 palsy after cervical laminoplasty using C5 nerve root ultrasonography. J Neurosurg Spine 2018;29:365-70. [Crossref] [PubMed]
- Sasai K, Saito T, Akagi S, et al. Preventing C5 palsy after laminoplasty. Spine (Phila Pa 1976) 2003;28:1972-7. [Crossref] [PubMed]
- Imagama S, Matsuyama Y, Yukawa Y, et al. C5 palsy after cervical laminoplasty: a multicentre study. J Bone Joint Surg Br 2010;92:393-400. [Crossref] [PubMed]
- Nassr A, Eck JC, Ponnappan RK, et al. The incidence of C5 palsy after multilevel cervical decompression procedures: a review of 750 consecutive cases. Spine (Phila Pa 1976) 2012;37:174-8. [Crossref] [PubMed]
- Katsumi K, Yamazaki A, Watanabe K, et al. Can prophylactic bilateral C4/C5 foraminotomy prevent postoperative C5 palsy after open-door laminoplasty?: a prospective study. Spine (Phila Pa 1976) 2012;37:748-54. [Crossref] [PubMed]
- Tamiya A, Hanakita J, Nakanishi K, et al. Analysis of the postoperative palsy of upper extremities of the cases undergone spinous process-splitting laminoplasty without foraminotomy. Spinal Surgery 2005;19:321-8. [Crossref]
- Takenaka S, Hosono N, Mukai Y, et al. The use of cooled saline during bone drilling to reduce the incidence of upper-limb palsy after cervical laminoplasty: clinical article. J Neurosurg Spine 2013;19:420-7. [Crossref] [PubMed]
- Kurosa Y, Yamaura I, Nakai S. Pathophysiology of postoperative C5 nerve root palsy. Sekitsui-Sekizui 1993;6:107-14.
- Shiozaki T, Otsuka H, Nakata Y, et al. Spinal cord shift on magnetic resonance imaging at 24 hours after cervical laminoplasty. Spine (Phila Pa 1976) 2009;34:274-9. [Crossref] [PubMed]
- Kaneyama S, Sumi M, Kanatani T, et al. Prospective study and multivariate analysis of the incidence of C5 palsy after cervical laminoplasty. Spine (Phila Pa 1976) 2010;35:E1553-8. [Crossref] [PubMed]
- Sodeyama T, Goto S, Mochizuki M, et al. Effect of decompression enlargement laminoplasty for posterior shifting of the spinal cord. Spine (Phila Pa 1976) 1999;24:1527-31; discussion 1531-2. [Crossref] [PubMed]
- Ramos MRD, Liu G, Tan JH, et al. Risk factors for surgical complications in the management of ossification of the posterior longitudinal ligament. Spine J 2021;21:1176-84. [Crossref] [PubMed]
- Hojo Y, Ito M, Abumi K, et al. A late neurological complication following posterior correction surgery of severe cervical kyphosis. Eur Spine J 2011;20:890-8. [Crossref] [PubMed]
- Chiba K, Toyama Y, Matsumoto M, et al. Segmental motor paralysis after expansive open-door laminoplasty. Spine (Phila Pa 1976) 2002;27:2108-15. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


