Chest CT abnormalities and quality of life: relationship in adult cystic fibrosis
Introduction
Patient reported outcomes (PRO) have become an important part of health outcomes research in adult cystic fibrosis (CF) (1). Their application is evidenced in many aspects of CF care including assessing new and established treatments and the cost-effectiveness of such interventions (2). Several specific tools have been developed for CF, the most validated contemporary instrument being the Cystic Fibrosis Questionnaire-Revised (CFQ-R) (3,4). The revised version has undergone rigorous psychometric testing and has been validated in numerous cross-sectional and longitudinal studies in CF (5-7). It has been widely applied to symptom comparisons between infectious lung pathogens, physical training programs, health resource utilization and treatment response in drug trials (8-12).
Chest CT has become widely accepted as a valuable imaging tool for the detection and monitoring of CF lung disease (13-15). Many centers obtain annual or biennial chest CT to monitor lung disease severity, but how chest CT abnormalities relate to how patients actually feel is currently unknown. Put another way, when a radiologist reports and grades abnormalities such as bronchiectasis or mucus plugging, how does that relate to how that patient with CF is actually feeling? A recent study examining this relationship found several significant associations between chest CT structural abnormalities and several CFQ-R domains (16). The purpose of our study was to further evaluate this relationship (I) specifically in an adult population with CF and (II) assess whether Inpatient versus Outpatient status influenced the findings.
Materials and methods
Patient population
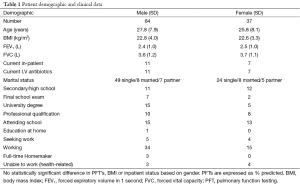
We prospectively enrolled 101 consecutive patients undergoing HRCT 15 years of age and older (mean age 27.8±7.9 years, range 15–48 years, 64 males, 37 females) in this CFQ-R questionnaire study. All patients had documented clinical, radiological and genotypic features of CF as well as an abnormal sweat test (sweat sodium and chloride >60 mmol/L). Age, body mass index (BMI), spirometry, history of tuberculosis, Pseudomonas cepacia and Aspergillus infection were recorded within 6 months of the HRCT. Demographic data and clinical parameters are detailed in Table 1. The institution’s hospital ethics committee approved the study and all patients gave written informed consent.
Full table
Health-related quality of life questionnaire
The CFQ-R is a disease-specific health-related quality of life questionnaire containing both generic and CF-specific scales and measures functioning during the previous 2 weeks (3,5). There are 12 domains including Physical Functioning, Emotional Functioning, Social Functioning, Body Image, Eating Problems, Treatment Burden, Respiratory Symptoms, Digestive Symptoms, Vitality, Health Perception, Weight and Role Functioning. Each CFQ-R scale yields standardized scores ranging from 0 to 100; higher scores indicate better QOL. The age-appropriate teen/adult version of the CFQ-R was used. The questionnaire has been previously been validated in several European CF populations (17-20). Patients were given the questionnaire when they attended for their CT, but were allowed to bring the questionnaire home if they wished and then send it back in.
HRCT protocol and scoring
Images were obtained using 64-slice CT (Sensation 64, Siemens, Erlangen, Germany) with patients in the supine position. Inspiratory images were obtained in suspended deep inspiration with 1mm slice thickness every 10 mm from the apices to the costophrenic angles. Expiratory images were obtained in full expiration at five levels: the top of the aortic arch, the carina, pulmonary veins, between level 3 and 5 and 2 cm above the diaphragm. Scanning parameters were 80–120 kVp and 50–120 mA with images reconstructed using a high spatial frequency bone algorithm and a 512×512 matrix. Lung windows with a width of 1,500 and level of −700 were applied.
Image analysis
Images were prospectively scored in random order by two chest radiologists (JD, AK) blinded to patient identification, clinical severity of disease, spirometry and questionnaire results. Images were scored using the modified Bhalla scoring system (21); which has been devised to evaluate the severity of CF in an objective manner. The Bhalla scoring system defines the severity of bronchial dilation and thickening relative to the adjacent pulmonary artery, and other parameters are scored according to the number of bronchopulmonary segments involved. This scoring system has been modified many times.
Abnormalities were defined according to recommendations of the nomenclature committee of the Fleischner society (22). Total CT score was derived by adding the scores for each abnormality, and ranged from 0–37. Absolute total scores were converted to percentages of the potential total maximum score.
Pulmonary function testing (PFT) (Vivaysis/Jaeger Masterscope System, Würzburg, Germany) was performed within 3 weeks before or after HRCT with the majority (78 of 101 scans, 77%) being performed on the same day. Measurements were obtained using described techniques, and predicted normal values were used to calculate percentage predicted values (23). Spirometry was expressed as percent predicted.
Statistical analysis
Patient demographics are provided as the mean ± standard deviation. Higher CT scores indicate worsening appearances whilst higher CFQ-R scores indicate better quality of life. Analysis was performed for inpatients and outpatients separately as well as for the entire group. Comparisons between groups were performed using the independent t-test. Multiple linear regression analysis was performed for each of the 12 CFQ-R domains as dependent variables and all clinical, spirometric and chest CT abnormalities as independent variables. Chest CT abnormalities in less than 5 patients were excluded from the analysis. All statistical analyses were performed with SPSS PASW Statistics 18 (SPSS Inc., Chicago, IL, USA). P values less than 0.05 were considered to indicate a significant difference.
Results
Eighteen patients were current inpatients at the time of the questionnaire and all were receiving intravenous antibiotics for pulmonary exacerbations. They also had a higher number of admissions in the last 12 months compared to outpatients (P<0.0001). There was no significant difference in prevalence of intravenous therapy between inpatients and outpatients or in atypical colonizing organisms such as Mycobacterium Avium intracellulare, Aspergillosis or Burkholderia Cepacia. Inpatients had significantly worse FEV1 (1.8 vs. 2.5, P<0.001) and BMI (21.5 vs. 23.5, P<0.001) compared to the outpatients group.
CT scores
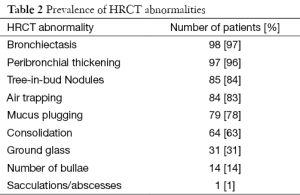
Mean percentage CT total score was 36.3±12 (range, 2.7–64.9) with the majority of patients scoring in the intermediate category (30–60%). Three patients had scores >50%. The most prevalent parenchymal abnormality was bronchiectasis (97%), followed by peribronchial thickening (96%) and tree-in-bud nodules (84%) (Table 2). Total CT score for inpatients was significantly worse than for outpatients (42.3 vs. 37.3, P<0.002).
Full table
Health-related quality of life scores
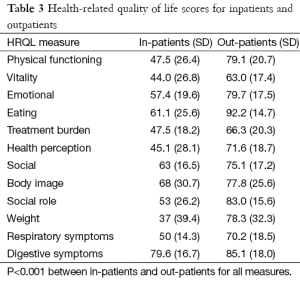
There was a wide spectrum of education within the cohort, with nine patients completing secondary school exams and 20% obtaining a college degree. Fifty percent of patients were actively working at the time of the study. For inpatients, Weight and Vitality scored the lowest whereas for outpatients, Vitality and Treatment Burden scored the lowest (Table 3). Significantly better scores were found in outpatients compared to inpatients for all domains (P<0.001 for all domains).
Full table
Regression analysis
Eating Problems, Treatment Burden, Health Perception and Body Image showed significant associations with several CT abnormalities but no clinical or spirometric variables (Table 4). Inpatients vs. outpatients status significantly influenced the models. For inpatients (Table 4), CT abnormalities were significantly (P<0.005 for all associations) associated with Respiratory Symptoms (Air Trapping), and also with Social Functioning (Consolidation) and Role Functioning (Consolidation). FEV1 was the only significant association with Physical Functioning.

Full table
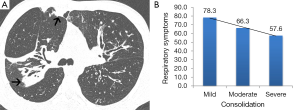
For outpatients (Table 4), CT abnormalities were significantly (P<0.005 for all associations) associated with Respiratory Symptoms (Consolidation) and also with Physical Functioning (Consolidation), Vitality (Consolidation, Severity of Bronchiectasis), Eating Problems (airway wall thickening), Treatment Burden (Total CT Score), Body Image (Severity of Bronchiectasis and Role Functioning (Tree-in-bud nodules). Consolidation was the commonest independent CT predictor for both inpatients (predictor for 2 domains) and outpatients (predictor in 3 domains) (Figure 1).
Discussion
The major findings from our study are that in a specifically adult CF population, chest CT abnormalities (I) are independently associated with the CFQ-R, (II) show stronger associations than traditional parameters such as FEV1 and BMI and (III) are associated with several CFQ-R domains besides respiratory symptoms. Inpatient vs. outpatient status appeared to significantly affect the findings, although we acknowledge the smaller proportion of inpatients in this study. In our original hypothesis we predicted chest CT abnormalities would correlate most strongly with the Respiratory Symptoms domain. In fact, only two CT abnormalities (air trapping and consolidation) were independent predictors of this domain. Importantly, clinical and spirometric variables were excluded from our regression model when chest CT abnormalities were included. Such findings highlight two points, firstly the importance of even small subsegmental areas of consolidation in influencing Respiratory Symptoms, and secondly the importance of including expiratory chest CT sequences in routine CF imaging protocols to evaluate for air trapping. How consolidation and air trapping might contribute to respiratory symptoms is of utmost importance. In other disorders such as chronic obstructive pulmonary disease (COPD) and asthma, air trapping has been shown to impair ventilator mechanics, increase airway resistance and increase elastic load, all factors known to have detrimental effects on normal respiratory physiology (24-27). The significance of pneumonia is well known to result in a decrease in normal gas exchange and to stimulate pulmonary receptors and C-fibers, two major mechanisms contributing to respiratory symptoms (28,29).
Similar findings to our own were found in a recent study by Tepper et al. who assessed the relationship between chest CT abnormalities and QOL in CF (16). They also found air trapping and opacities to correlate significantly with Respiratory Symptoms. In contrast, they found on univariate analysis that both mucus plugging and airway wall thickening were associated with Respiratory Symptoms. The older age of our adult CF patient group is likely to explain the difference in results, since older patients are more prone to pulmonary infections (30). It may also be due in part to different CT scoring systems, as our study utilized the modified Bhalla score whereas the study by Tepper et al. utilized the Brody scoring system. It is worth emphasizing that the most important associations in both analyses for many CFQ-R domains were generally acute inflammatory abnormalities such as consolidation, sacculations/abscesses, tree-in-bud nodules and airway wall thickening. Such abnormalities are typically the most reversible (31), and since questionnaire responses may also show changes in response to treatment (7), it is intuitive that reversible CT abnormalities are also the ones most closely associated with health related QOL measures.
A somewhat unexpected finding was the association between chest CT abnormalities and domains other than Respiratory Symptoms, such as Physical Functioning, Vitality and Treatment Burden. The mechanisms influencing how CF lung disease might impact these non-respiratory QOL aspects are not strongly elucidated in CF. In other pulmonary disorders such as COPD, pulmonary exacerbations have been shown to influence many non-respiratory QOL aspects, for example physical activity and treatment burden (32). The perception of fatigue has also been shown to be strongly influenced by respiratory disease in COPD (33,34). Breslin et al. (34) have shown associations between deteriorating lung function and both fatigue and physical activity in COPD. It may be that, similar to COPD, in CF deteriorating respiratory disease results in physical deconditioning which in turn results in deterioration in many aspects of QOL. It is also known that lung disease effects the prevalence and severity of depression in CF (35), which in turn affects aspects such as social and role functioning. It is clear that the non-respiratory domains of the CFT-R are highly complex with multifactorial etiologies, and although our study was not designed to elucidate these, we suggest it corroborates previous work showing that the systemic sequelae of chronic respiratory inflammation may be more pervasive than confined to the respiratory system alone.
In our analysis several chest CT abnormalities showed stronger associations with the CFQ-R than traditional health outcomes measures such as FEV1 and BMI. For example, only chest CT abnormalities were associated with Respiratory Symptoms, irrespective of inpatient or outpatient status. Several additional CFQ-R domains (Eating Problems, Treatment Burden, Health Perception, Body Image) showed associations on multiple regression analysis with chest CT abnormalities but not FEV1 or BMI. Traditionally, chest CT has not been included in studies assessing the CFQ-R, although there are many that have evaluated the psychometric properties of the CFQ-R in relation to traditional health measures such as FEV1 and BMI (5). In the majority of studies, FEV1 correlates modestly with Respiratory Symptoms. Despite being wholly different in design and size to these previously published studies, our study on chest CT abnormalities excluded both FEV1 and BMI from the regression model for respiratory symptoms. Such findings highlight the potential utility of chest CT in QOL studies in CF, in which CT abnormalities may influence traditional prediction models of QOL such as the CFQ-R.
Several limitations to the current study should be noted. This study was not designed to evaluate prognosis, but rather to assess whether chest CT abnormalities are associated with how CF patients are feeling. We included teen/adults in our cohort, and our results should be interpreted carefully when extending the findings to a pediatric population. It was not our purpose to validate the CFQ-R, such validations have been previously performed by others (3,4,6).
In conclusion, chest CT abnormalities are significantly associated on multiple regression analysis with many aspects of QOL in CF, independent of clinical and spirometric parameters. Consolidation and air trapping are most strongly associated with Respiratory Symptoms. Our findings provide support for the inclusion of chest CT in QOL studies in CF.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zemanick ET, Harris JK, Conway S, et al. Measuring and improving respiratory outcomes in cystic fibrosis lung disease: opportunities and challenges to therapy. J Cyst Fibros 2010;9:1-16. [Crossref] [PubMed]
- Goss CH, Quittner AL. Patient-reported outcomes in cystic fibrosis. Proc Am Thorac Soc 2007;4:378-86. [Crossref] [PubMed]
- Quittner AL, Buu A, Messer MA, et al. Development and validation of the Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest 2005;128:2347-54. [Crossref] [PubMed]
- Yuksel H, Yilmaz O, Dogru D, et al. Reliability and validity of the Cystic Fibrosis Questionnaire-Revised for children and parents in Turkey: cross-sectional study. Qual Life Res 2013;22:409-14. [Crossref] [PubMed]
- Quittner AL, Sawicki GS, McMullen A, et al. Psychometric evaluation of the Cystic Fibrosis Questionnaire-Revised in a national sample. Qual Life Res 2012;21:1267-78. [Crossref] [PubMed]
- Sawicki GS, Rasouliyan L, McMullen AH, et al. Longitudinal assessment of health-related quality of life in an observational cohort of patients with cystic fibrosis. Pediatr Pulmonol 2011;46:36-44. [Crossref] [PubMed]
- Quittner AL, Modi AC, Wainwright C, et al. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135:1610-8. [Crossref] [PubMed]
- Briesacher BA, Quittner AL, Saiman L, et al. Adherence with tobramycin inhaled solution and health care utilization. BMC Pulm Med 2011;11:5. [Crossref] [PubMed]
- Oermann CM, Retsch-Bogart GZ, Quittner AL, et al. An 18-month study of the safety and efficacy of repeated courses of inhaled aztreonam lysine in cystic fibrosis. Pediatr Pulmonol 2010;45:1121-34. [Crossref] [PubMed]
- Quittner AL, DiGirolamo AM, Michel M, et al. Parental response to cystic fibrosis: a contextual analysis of the diagnosis phase. J Pediatr Psychol 1992;17:683-704. [Crossref] [PubMed]
- Retsch-Bogart GZ, Quittner AL, Gibson RL, et al. Efficacy and safety of inhaled aztreonam lysine for airway pseudomonas in cystic fibrosis. Chest 2009;135:1223-32. [Crossref] [PubMed]
- Briesacher BA, Quittner AL, Fouayzi H, et al. Nationwide trends in the medical care costs of privately insured patients with cystic fibrosis (CF), 2001-2007. Pediatr Pulmonol 2011;46:770-6. [Crossref] [PubMed]
- Rowe SM, Borowitz DS, Burns JL, et al. Progress in cystic fibrosis and the CF Therapeutics Development Network. Thorax 2012;67:882-90. [Crossref] [PubMed]
- Mott LS, Park J, Gangell CL, et al. Distribution of early structural lung changes due to cystic fibrosis detected with chest computed tomography. J Pediatr 2013;163:243-8.e1-3.
- de Jong PA, Nakano Y, Lequin MH, et al. Progressive damage on high resolution computed tomography despite stable lung function in cystic fibrosis. Eur Respir J 2004;23:93-7. [Crossref] [PubMed]
- Tepper LA, Utens EM, Caudri D, et al. Impact of bronchiectasis and trapped air on quality of life and exacerbations in cystic fibrosis. Eur Respir J 2013;42:371-9. [Crossref] [PubMed]
- Olveira G, Olveira C, Gaspar I, et al. Validation of the Spanish version of the Revised Cystic Fibrosis Quality of Life Questionnaire in adolescents and adults (CFQR 14+ Spain). Arch Bronconeumol 2010;46:165-75. [Crossref] [PubMed]
- Groeneveld IF, Sosa ES, Pérez M, et al. Health-related quality of life of Spanish children with cystic fibrosis. Qual Life Res 2012;21:1837-45. [Crossref] [PubMed]
- Schmidt A, Wenninger K, Niemann N, et al. Health-related quality of life in children with cystic fibrosis: validation of the German CFQ-R. Health Qual Life Outcomes 2009;7:97. [Crossref] [PubMed]
- Bregnballe V, Thastum M, Lund LD, et al. Validation of the Danish version of the revised cystic fibrosis quality of life questionnaire in adolescents and adults (CFQ-R14+). J Cyst Fibros 2008;7:531-6. [Crossref] [PubMed]
- Judge EP, Dodd JD, Masterson JB, et al. Pulmonary abnormalities on high-resolution CT demonstrate more rapid decline than FEV1 in adults with cystic fibrosis. Chest 2006;130:1424-32. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012;185:435-52. [Crossref] [PubMed]
- O'Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis 1993;148:1351-7. [Crossref] [PubMed]
- O'Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:770-7. [Crossref] [PubMed]
- Lougheed MD, Fisher T, O'Donnell DE. Dynamic hyperinflation during bronchoconstriction in asthma: implications for symptom perception. Chest 2006;130:1072-81. [Crossref] [PubMed]
- Harty HR, Mummery CJ, Adams L, et al. Ventilatory relief of the sensation of the urge to breathe in humans: are pulmonary receptors important? J Physiol 1996;490:805-15. [Crossref] [PubMed]
- Undem BJ, Nassenstein C. Airway nerves and dyspnea associated with inflammatory airway disease. Respir Physiol Neurobiol 2009;167:36-44. [Crossref] [PubMed]
- LiPuma J. The new microbiology of cystic fibrosis: it takes a community. Thorax 2012;67:851-2. [Crossref] [PubMed]
- Shah RM, Sexauer W, Ostrum BJ, et al. High-resolution CT in the acute exacerbation of cystic fibrosis: evaluation of acute findings, reversibility of those findings, and clinical correlation. AJR Am J Roentgenol 1997;169:375-80. [Crossref] [PubMed]
- Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418-22. [Crossref] [PubMed]
- Baghai-Ravary R, Quint JK, Goldring JJ, et al. Determinants and impact of fatigue in patients with chronic obstructive pulmonary disease. Respir Med 2009;103:216-23. [Crossref] [PubMed]
- Breslin E, van der Schans C, Breukink S, et al. Perception of fatigue and quality of life in patients with COPD. Chest 1998;114:958-64. [Crossref] [PubMed]
- Riekert KA, Bartlett SJ, Boyle MP, et al. The association between depression, lung function, and health-related quality of life among adults with cystic fibrosis. Chest 2007;132:231-7. [Crossref] [PubMed]


