Precision medicine in myasthenia graves: begin from the data precision
Introduction
The concept of precision medicine is being accepted more prevalently in medical and health care world, which is defined as treatments targeted to the needs of individual patients on the basis of genetic, phenotypic, or psychosocial characters that distinguish a given patient from other patients with similar clinical presentations (1). Its aim is to improve clinical outcomes and minimize unnecessary side effects for individual patients. The scope of precision medicine is rapidly expanding by refining the classification of disease, often with important prognostic and treatment implications.
Myasthenia gravis (MG) is a prototypic autoimmune disease, with known autoantigens, such as acetylcholine receptor (AChR), muscle-specific kinase (MuSK) or LDL receptor-related protein 4 (LRP4), and relevant antibody-mediated autoimmune response, leading to fatigue and weakness of skeletal muscles. These antigens are proteins at the neuromuscular junction (NMJ), which maintain the structure of motor synapse and facilitate neuromuscular conduction of motor impulses. MG subgroups with autoantibodies against them have been found to be with relatively distinct clinical features (2-4). Functional blocking antibodies and tissue-damaging antibodies of different subclass (IgG1, IgG3 or IgG4) against various epitopes of the autoantigens have been reported (2,3). Severity of MG was found to be correlated with antibody levels with conflicting evidence, partially due to the intrinsic mechanism of different antibodies, difference in methods of antibody testing, and the measurement of severity. Thymus abnormalities are often found in MG patients, with different presumed mechanism between hyperplasia and thymoma (5). Clinical and immunological heterogeneity exists in MG as a result of all these factors. Genetic predisposition of MG is now an active research area with the aim to explore a risk marker or a potential specific treatment target (6-8). The genes of both immune-modulating proteins (e.g., HLA or cytokines) and autoantigens (9) have been found to be associated with MG. The therapeutic effects in MG was determined not only by the immunological treatment, but also by adherence to treatment, incidental worsening factors (e.g., infection and emotional stress), as well as by genetic background of individuals. Due to all these factors, the data of MG is far from individually precise now, partially due to the rarity and heterogeneity of this disease. In this manuscript, we discuss the precision medicine in this chronic autoimmune disease with seemingly ambiguity.
Relative precision in the main domains of myasthenia gravis (MG) data
In MG, important data on clinical and immunological phenotypes could be classified into several domains: (I) demographic: gender and onset age; (II) presenting symptoms: ocular, bulbar and other non-ocular muscles; (III) the first involved non-ocular muscle group in ocular presenting patients and the interval between onset and generalization; (IV) thymus status; (V) pathogenic autoantibodies against AChR, MuSK or LRP4, and other relevant autoantibodies (e.g., against striated muscle antigens, titin and ryanodine receptor [RyR]); (VI) muscle involvement and severity at sample collection and at the maximum worsening; (VII) response to specific treatment.
Gender is the most precise data, while onset age might be confused by ambiguous judgment of MG symptoms. The first symptom in a mild episode might be forgot or unrecognized by the patients due to spontaneous remission and fluctuation of symptoms. Some concurrent unspecific symptoms might confuse both the patient and neurologist. In our recent study, we examined the patients with fatigue test and neostigmine test immediately after screening with a questionnaire for ongoing symptoms. Half of the involved muscles judged by an experienced neurologist changed. Fatigue test may reveal muscle involvement which was not reported by the patients. After neostigmine test, fatigue in some muscle groups was found to be caused by other diseases, especially in neck and limb muscles by cervical spondylosis, lumbar disease, or nerve root lesions (10). Therefore, the precise identification of onset age is related to accurate judgment on MG symptoms. Previous fluctuating symptoms should be sought by careful screening in each muscle group (ocular, facial, bulbar, neck, limb girdle, limb extremities, and respiratory muscles) with standardized questionnaires and medical records. For the patients who cannot provide medical records, timing of the first outpatient visit due to the presenting symptoms could be viewed as the onset. This principle also applies to identification of the first non-ocular muscle involvement in determining the time of generalization in patients presented with ocular symptoms.
The role of thymoma and diagnostic autoantibodies becomes more important in MG phenotypes. However, the accuracy of their information might be hampered by suboptimal antibody testing and evaluations of the thymus. MG with thymoma is considered as a paraneoplastic syndrome, which has different clinical and immunological features and different mechanisms compared with non-thymoma patients (5,11). Current guidelines of MG treatment do not recommend thymectomy in ocular MG and AChR antibody negative patients (12-14). Thus, pathological data on thymus is not available in all MG patients. The pathological confirmed thymoma is accurate in individual patients, but might lead to selection bias in population-based association studies due to various proportions of MG patients who are not advised by treating neurologists or decline to receive thymectomy. Although CT scan is considered as the main screening tools for thymoma, with high sensitivity and specificity, thymic hyperplasia is sometimes misdiagnosed as thymoma solely on CT imaging, while small thymoma beyond the resolution of imaging might be missed (15). MRI does not add the sensitivity in the screening of thymoma (16). Moreover, new thymus abnormality could be found several years after the initial negative CT scan (17). Therefore, other laboratory investigations should be employed to search for potential thymoma. Antibodies against striated muscle antigens (titin and RyR) and radiological examination of are similar sensitivity for the presence of thymoma in MG. Presence of such antibodies in MG patients younger than 60 years strongly suggests a thymoma, while absence of them at any age strongly excludes thymoma (11). The combination of repeated CT scan and testing of titin and RyR antibodies could be the bases of non-invasive screening of thymoma. 99mTc-MIBI single photon emission computed tomography (SPECT) have been used for assessing anterior mediastinal mass and distinguishing the grade of malignancy of thymic epithelial tumors (18). In a preliminary study, we compared the 99mTc-MIBI image and pathology of thymus in 38 MG patients, and found prominent uptake of MIBI in thymoma, while weak uptake in thymus hyperplasia (unpublished data). This examination might be used as an additional tool to screen thymoma, especially ectopic thymoma.
Pathogenic autoantibodies against NMJ proteins are regarded as the most important biomarkers in MG, not only because relative distinct clinical features of MG were found to be linked to the presence of specific antibodies, but also because levels of some antibodies were found to be correlated with the severity of MG (2,19-21). The positivity of antibodies depends on several factors: (I) The antigens employed and the testing methods used. Take AChR antibody for example, AChR derived from a genetic modified rhabdomyosarcoma cell line or denervated human muscle are often used as antigens in commercial kits [enzyme-linked immunosorbent assay (ELISA) and radioimmunoprecipitation assay (RIA)]. More sensitive cell-based assays allow detection of antibodies that only bind to AChRs clustered on the surface of mammalian cells (22). The positivity also depends on the cut-off values of quantitative testing such as ELISA and RIA, and on detection threshold of qualitative testing in different laboratories; (II) repeated AChR antibody testing. Positive results may be found in the patients with formerly negative results, especially in milder patients with shorter duration of disease (23,24). This phenomenon was found in other autoimmune disease, for example, in a proportion of multiple sclerosis patients, oligoclonal bands could become positive in repeated testing several months after the first negative testing (25); (III) effects of treatment on antibody levels. In MG, once the antibodies appear, they tend to be positive consistently even their level decrease after effective treatment with potent immunosuppressive agents or thymectomy (26-30). This may be related to persistence of long-lived plasma cell (31). Minor difference in the positivity might be found due to variation of antibody levels around the testing threshold in a small proportion of tested subjects. If the first sample is tested as negative after effective treatment, it is better to retest the antibodies when there is a relapse. The testing reports should include the methods (quantitative or qualitative), cut-off value for positivity in quantitative testing and the original results.
Clinical severity is defined in different ways in various studies: (I) simple classes based mainly on muscle involvement range and impairments, which can be assessed with patient-reported motor functions and simple beside examinations, such as Osserman classes (32), MGFA classes (33) and Oosterhuis score (34,35). The former two are in fact directly modified from clinical classification systems of MG, without primary aim to evaluate the severity. Their value in the measurement of severity was questioned (33). The last one is based on patient-reported impairment of daily livings, designed in the similar reasoning of the former. The interobserver agreements of Osserman and MGFA classification systems were found to be fairly good in a well representative cohort of 64 MG patients with various involvement range and severity. The agreements of Osserman classes were found a little better than those of MGFA classes, partially due to more categories and relative vague definition of severity in generalized types of MGFA classes (10). The MGFA recommendation also pointed that disagreement might exist even between two MG experts (33). The Osserman and MGFA classification system are based on the general impression of physicians on involvement and severity. Hence, using them as the hallmark of severity is a vicious circle; (II) composite ordinal scales such as Quantitative Myasthenia Gravis score (QMGs) (36), Myasthenia Gravis Composite (MGC) (37), Myasthenic Muscle Scale (MMS) (38), which are commonly used in Western countries, and the Absolute and Relative Score of MG (ARS-MG) (39), a commonly used scale in China. In 60 representative MG patients, all four scales were found to have good internal consistency, test-retest reliability, interobserver reliability, and construct validity. There were strong correlation between QMGs as criterion and each of the other three scales. Moderate to strong correlations were also found between each of these scales (40). The construct validity of scales, the good reliability of each scales and the intrinsic sensitivity of the scores in the measurement of severity endow all these scales with good performance and correlations. Nevertheless, these scales are composite ordinal scales, which mean that a score of five is not truly five times of a score of one in the measurement of severity. Rasch method might solve this problem. This method has been used in the development of severity scales in neuromuscular diseases (41,42), and began to be explored in MG (43).
Muscle involvement and severity during the maximum worsening reflect the clinical features and prognosis comprehensively, hence it is used in subgroup classification of MG. MG is a chronic autoimmune disease with characteristic daily fluctuations due to compromised NMJ conduction, so the severity of MG on a given follow-up may vary dependent on the intensity of immunological reactions, concurrent pathophysiological factors (infection, fatigue, stress, fever or menstruation) on NMJ and the effects of cholinesterase inhibitors. The clinical severity during the maximum worsening without concurrent pathophysiological factors is the best reflection of immunological intensity. Although immunosuppressive treatment may prevent generalization worsening of MG, the maximum severity is seemingly determined by genetic factors, as reflected by the facts that maximum severity is attained after several relapses, regardless of potent immunosuppressive treatment. This is also supported by natural history studies that generalization from ocular MG and worsening to the maximum severity tend to occur during the initial 2 years after onset (44). Therefore, clinical severity measured during the maximum worsening during the long-term follow-up will provide endophenotype information for MG research. Muscle involvement and severity at sample collection are also important endophenotypes to be correlated with profiles and levels of antibodies and other biomarkers (e.g., cytokines or acute phase proteins). Care should be taken that quantitative measurement of clinical severity is better performed at least 6 h after the last dose of cholinesterase inhibitors in order to eliminate the effects of them (45). Moreover, all patients should be devoid of the influence of over fatigue or hunger when examining. The patients should be encouraged to cooperate fully in similar efforts in each follow-up to minimize the variability due to less cooperation.
Concurrent autoimmune diseases are relatively distinct in MG, with variation in different subgroups (46). Non-motor symptoms such as pure red cell aplasia, alopecia areata and immunodeficiency in MG are often overlooked, some of which might be mediated by cellular immunity, different from typical symptoms mediated by pathogenic autoantibodies (47). This information should also be recorded.
Treatment response is an important feature of MG and a direct variable in precision medicine. Sensitive or insensitive to a given treatment is determined by the study duration, outcome measures, concurrent treatment, baseline features and analysis designs. Study should be long enough to ensure adequate immune-modulation to take effects. Shorter study duration as the cause of failure in clinical trial of MG has been discussed (48). Optimal outcome measure is still in its infancy in the field of MG. Changes of QMGs is the most adopted definition, with scores improvement of more than 3 or decreasing to 0 as clinical meaningful (49,50). Minimal clinically important difference has been established for clinical trials of MG (51). Relative change of the absolute MG severity scores is an individualized measure of treatment response. In China, the relative score of MG severity is commonly adopted. It is defined as (pre-treatment absolute score—post-treatment absolute score)/pre-treatment absolute score (39). The improvement index with similar formula has been used in other autoimmune diseases (52). However, the cut-offs of response magnitude are still arbitrary. Moreover, the cut-off value for a long-term treatment response might not be the same as for the short-term response. MG is often treated with more than one single immunosuppressive agent. Hence, the response of add-on therapy should be adjusted with the cumulative effects of other treatments. The studied treatment and/or the concurrent treatment might be tapered or increased with the change of severity. Bayesian method is a good tool to evaluated the cumulative effects of treatment and other confounding factors (including the untreated duration), and has been used in the studies of other autoimmune diseases such as multiple sclerosis (53), but not yet in MG. Propensity score has been used in the stratification in clinical trials of MG (54). Whether such a score which composed of baseline clinical and immunological features of MG could be used in the association studies has not been studied. Clinical trials are often designed in cohort studies with the first outcome event as the main endpoint, but the association studies are always designed in case-control studies. The cohort design is often for group comparison, the responders defined in this design might introduce bias in case-control design due to various treatment durations in different responders. For the evaluation of a short-term treatment in the treatment-naive patients, the changes of severity scores at a pre-specified time after treatment could be used for the definition of response. For a long-term treatment, the postintervention status (33) could be used as the index of response. The patients withdrawn after beginning the treatment should be followed and determined as responder or non-responder. For patients who request to add plasma exchange, intravenous immunoglobulin or other immunosuppressive agents, or have to receive them as rescuing treatment, the severity at the end of studied duration may help to determine the response. Sensitivity analysis should be performed by including these patients after the analysis of patients who finished the treatment per protocol.
The aggravation speed of MG severity in the initial 3 months might be a risk factor for myasthenic crisis and treatment-refractory courses. One solution for optimal calculation of aggravation speed is to derive a ratio of the QMGs and time from onset as the progression index. However, the fact that QMGs is not linear limits this approach, which is similar to EDSS in multiple sclerosis. An individualized score based on algorithm adjusting disability for disease duration has been developed and was found associated with the prognosis of multiple sclerosis (55,56), but not yet in MG. The delay of transition from ocular involvement to generalized MG might also be an important endophenotype for ocular-presenting patients, especially in the untreated patients.
Subgroups of myasthenia gravis (MG)
Subgroups in a heterogeneous disease provide good bases for precision medicine. The early subgrouping systems of MG were based on muscle involvement range and severity, such as in Osserman (32) and MGFA (33) classification systems. These clinically based systems aim to recognize the impairment and natural history of MG, without considering the pathogenesis and potential treatment targets of MG. In recent years, subgrouping schemes have been established based on the combination of clinical, immunological and pathological data.
Differences in onset age, muscle involvement (ocular, bulbar and generalized), antibody profiles [including pathogenic antibodies (AChR, MuSk, LRP4), anti-striated muscle antibodies (titin and RyR)], and thymic pathology (thymoma, thymus hyperplasia) are the bases of modern classification, with consideration for the association with other organ-specific autoimmune response, clinical severity and response to immunosuppressive agents and thymectomy as references. Various classification systems have slight difference in their definitions. The system of Meriggioli and Sanders (19) includes generalized MG (without thymoma, subdivided into early-onset and late-onset subgroups), ocular MG, thymoma MG, generalized MG without AChR antibody and seronegative MG (lack both AChR and MuSK antibodies). The boundary between early and late onset is 40 years. The system of Berrih-Aknin (20) includes: pure ocular MG, generalized MG with AChR antibodies (subdivided into early onset MG and late onset MG), MG without AChR antibodies (subdivided into MuSK antibody MG, LRP4 antibody MG and clustered AChR antibody MG). This system depends much on the pathogenic antibodies and does not specify a subgroup with thymoma. The system of Gilhus and Verschuuren (21) includes: non-thymoma MG (AChR antibody positive, subdivided into early-onset MG and late-onset MG), thymoma MG, MuSK associated MG, LRP4 associated MG, antibody-negative generalized MG, and ocular MG. In all three systems, the definition of ocular MG required a follow-up of at least 2 years, citing that if the involvement remains limited to the ocular muscles after 2 years, there is 90% likelihood that the disease will not generalize (44). For the generalized subgroups, thymoma-related and antibody-related subgroups, once the data of muscle involvement, thymoma or specific antibodies is satisfied, the subgroup can be defined. Boundary between early and late onset in the latter two systems is 50 years (20,21). Relative distinct features were delineated in ocular MG, thymoma MG and antibody-associated MG, although overlap between each of these subgroups exists even in the same systems. The antibody negative subgroup is a heterogeneous group, patients are essentially indistinguishable from patients with AChR antibody positive MG in terms of clinical features, pharmacological treatment response, and even thymic abnormalities in some cases. Moreover, patients with pathogenic antibodies against more than one NMJ proteins (e.g., MuSK and LRP4) were found with improved testing methods, which makes some patients be allocated into more than one antibody associated subgroup (57-59). The present “standard” diagnostic testing might change in the future, making studies on subgroups with different testing methods incomparable. The above-mentioned difference in the definitions limits the comparisons and meta-analysis of studies with different subgrouping systems. Moreover, all three subgrouping systems did not consider the childhood MG. Juvenile MG (JMG) includes infants, children, and adolescents aged patients, without consensus on the boundary to early onset MG, although subdivision has been suggested according to the onset age as pre-pubertal (12 years) and post-pubertal (60).
Besides onset age, gender, antibodies and thymoma, initial muscle involvement (ocular/generalized) and maximum muscle involvement might also contribute to subgrouping. These elements are highly interrelated, which is shown in our studies that most elements were not independent in association with susceptibility and severity of MG due to interactions among these elements (61,62). A cluster analysis has been used to classify MG subgroups, putting as much elements as possible into an integral comprehensive analysis (63). Although boundary between early onset and late onset at 50 years was confirmed, the other combinations of elements in the previous subgrouping schemes were not found. Unfortunately, the detailed definition of each element was not provided in the study (63). Cluster analysis and principal analysis have been used in other autoimmune diseases to establish subgroups (64-66). Our group used data mining technique to explore subgroup clusters of MG and confirmed existence of some subgroups in the above-mentioned subgroup classifications (unpublished data). During this process, it is important to select the basic elements and to avoid ambiguous and overlapping definition of each element.
In the present time, we propose a basic subgrouping scheme as follows (Figure 1): (I) JMG, boundary to early onset MG should be further explored, with additional subdivision into pre-pubertal and post-pubertal as suggested (60); (II) adult MG with thymoma, pathology-confirmed or typical image suggested, with consideration of supplementary role of titin and RyR antibodies in implying or excluding potential thymoma in younger patients (11); (III) adult MG with AChR antibody but without thymoma, with repeated testing after an optimal interval (e.g., 1 year) in milder patients with shorter duration or previously treated patients, whose first testing is negative; (IV) adult MG without AChR antibody and thymoma. In group 3, further subdivision into early onset and late onset (50 years old), or into ocular presenting MG and generalized presenting MG. This will enable patients to be allocated into relative fewer subgroups with higher consistency, to avoid allocation a patients into several subgroups, and to facilitate comparison among different studies. This subgrouping scheme should be tailored according the study purpose in epidemiological, immunological and genetic researches and clinical trials, as well as in clinical practice. Subgrouping into MuSK or LRP4 associated MG could be taken in specific targeted researches prudently.
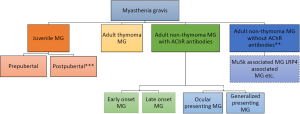
Quantitative traits in myasthenia gravis (MG): endophenotypes and intermediate phenotypes
Quantitative traits provide important clues to the pathogenesis and prognosis. In MG, severity and status of MG (e.g., post-intervention status) may serve as endophenotypes, while levels of antibodies and other biological molecules may serve as intermediate phenotypes.
The correlation between the levels of antibodies and clinical severity of MG is a long-term debate. AChR antibody level appears to provide an index of disease severity within the individual (67) and in group analysis of patients with shorter duration (68), while other studies did not confirmed this correlation. The conflicting results might be caused by the above-mentioned variations in severity definition and differences in testing methods. The quantification of the antibody levels is also important. Much attention should be paid on the units of antibody levels in the comparison among different studies. The levels might be expressed in original value as optic density (OD), nmol, ng, an inhibition rate, or as a log transformation of the maximum dilutions, depending on the testing methods. Take ELISA methods for example. Original OD value or the log transformation of the maximum dilutions are the best according to the principle of ELISA, while the values expressed in nmol or ng calculated with standard curve or empirical formula may introduce variation due to the complicated calculating process with several covariables. Modulating molecules involved in autoimmune diseases have been explored as biomarkers or surrogate markers, and used in association studies. For example, cytokine levels have been associated with cytokine gene polymorphism in MG (69). We now have a battery of target-specific modulating therapies in the treatment of MG (70). With the design of Mendelian randomization approach, the role of candidate modulating molecules has been confirmed in the pathogenesis of diseases, and has been assumed as treatment target of an existing agent (71). Therefore, the accurate quantification of these molecules is important in researches of precision medicine.
The storage conditions and freezing/thawing of stored samples are also important in precise measurement of antibodies and biological molecules. In a recent study, we confirmed that testing serum samples stored at room temperature, 4 °C and in frozen state and plasma samples stored in frozen state got consistent results of AChR antibody levels with a ELISA method, providing a base for using differently stored samples in the research (72). But for more unstable molecules, more care should be taken in the collecting and storage conditions (73). The effects of concurrent infections on testing results, especially on cytokines and acute reaction proteins, should be considered. Concurrent infections may aggravate autoimmune disease by innate immunity, and may lead to immediate changes in acute reaction proteins (74). After treatment, the change in antibody levels might introduce slight variation in antibody positivity and antibody levels. Immune modulating moleculars (e.g., cytokines) are even more easily to be affected by treatments. Therefore, the intermediate phenotypes should be tested in treatment-naive and infection-free patients.
Disease registries in the precision medicine of myasthenia gravis (MG)
Precision medicine research is in much debt to the development of disease registries. More and more disease-specific registries have been set up, relevant common element data (CDE) and guidelines on data sharing, sample collection and bioinformatics have been established (75,76). MG registries have been set up and began to showed their prominent contributions on recognizing the natural history, concurrent autoimmune disease profiles, outcome determinants and antibody profiles of MG (77,78). MG specific CDE has been provided under the scope of the NIH CDE collections (79). Although MGFA recommendation on MG research (33) has paved the path for advanced researches in MG, most of the proposal raised 15 years ago deal mostly with clinical research, with no specific consideration on the precision medicine. The NIH MG-CDE also does not mark the essential elements involved in the research of precision medicine. Nevertheless, the elements included in these two files cover almost all of the essential elements. We can extract the needed data by giving accurate definitions of the clinical and immunological elements, as well as subgroups, as the bases of phenotypes. Although MGFA recommendation (33) and NIH MG-CDE (79) included some information on thymus data, systematic thymus protocol including the radiology, surgery, staging, pharmacotherapy, sampling and pathological protocols should be found in other resources. The role of thymus in the pathogenesis of MG is becoming more prominent and the value of repeated surgery to treat MG is under great attention (80). Fortunately, the standard thymus protocols developed by the International Thymic Malignancy Interest Group provide detailed CDEs of thymus (81-86) and may lend their strength to MG researches.
Moreover, precise data of the qualified healthy control is also important in precision medicine. No such guideline on control information has been established yet in MG. In the meantime, we can learn ideas from the definitions and application guidelines for control groups in cerebrospinal fluid biomarker studies in multiple sclerosis (87).
Scientific bases of precise analysis
It is important to balance the data between precision in individual patients and the representativeness in patient population in the enrollment process. Selection bias is easily caused by insufficient follow-up in specific MG groups, such as child and the aged, the mildest and the most severe patients, patients with good response/spontaneous remission and devastating courses. Once the patients enter a MG registry, endeavor should be taken to ensure the patients be followed as longer as possible. The longer is the follow-up, the more precise is the data. We are trying to use a simplified scoring scale and a structured questionnaire which can be used by patients to ensure online follow-up for the patients. The patients, who lost follow-up in a consecutively enrolled cohort, should be checked with the frequencies of main clinical features of MG (onset age, gender, presenting symptoms, thymoma, and antibodies) to analyze the potential selection bias due to follow-up.
In the plan for performing a research in the precision medicine in the field of MG, systematic collection of data according to pre-specified CDEs is paramount. We wish our discussion will contribute the CDEs of our readers. In the analysis and report of research, relevant guidelines (88,89) and advice (90) should be considered. It should be born in mind that scientists are not immune to biased analysis process (hypothesis myopia, cherry-picked, etc.) (91). Moreover, statistical associations are far from causation. More sound evidence is waited to confirm a promising finding (92).
Conclusions and further directions
All precision medicines begin from the data precision. The precision of data will improve when more knowledge and experience from clinical research accumulate and are sorted in a logical rationality based on solicited facts and scientific reasoning. The common data elements of MG in precision medicine should be defined in detail and devoid of overlap and confounding factors. All these should be based on the comprehensive understanding of the pathogenesis, clinical and immunological features of MG, and of the principles and confounders of measurements in severity and levels of biomolecules. Advanced analysis such as cluster analysis and data mining would show their promising role in the future research. With the thoughts of the precision in individual data, in contrast to the relatively vague data in group comparison, the precision medicine will begin from prospective clinical studies and clinical registries of MG.
Acknowledgements
Funding: This work was supported by National Natural Science Foundation of China (No. 81070963) and Natural Science Foundation of Shandong Province (No. ZR2010HM019).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med 2015;372:2229-34. [Crossref] [PubMed]
- Verschuuren JJ, Huijbers MG, Plomp JJ, et al. Pathophysiology of myasthenia gravis with antibodies to the acetylcholine receptor, muscle-specific kinase and low-density lipoprotein receptor-related protein 4. Autoimmun Rev 2013;12:918-23. [Crossref] [PubMed]
- Keijzers M, Nogales-Gadea G, de Baets M. Clinical and scientific aspects of acetylcholine receptor myasthenia gravis. Curr Opin Neurol 2014;27:552-7. [Crossref] [PubMed]
- Berrih-Aknin S. Myasthenia Gravis: paradox versus paradigm in autoimmunity. J Autoimmun 2014;52:1-28. [Crossref] [PubMed]
- Marx A, Pfister F, Schalke B, et al. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun Rev 2013;12:875-84. [Crossref] [PubMed]
- Giraud M, Vandiedonck C, Garchon HJ. Genetic factors in autoimmune myasthenia gravis. Ann N Y Acad Sci 2008;1132:180-92. [Crossref] [PubMed]
- Zagoriti Z, Kambouris ME, Patrinos GP, et al. Recent advances in genetic predisposition of myasthenia gravis. Biomed Res Int 2013;2013:404053.
- Avidan N, Le Panse R, Berrih-Aknin S, et al. Genetic basis of myasthenia gravis, a comprehensive review. J Autoimmun 2014;52:146-53. [Crossref] [PubMed]
- Giraud M, Taubert R, Vandiedonck C, et al. An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus. Nature 2007;448:934-7. [Crossref] [PubMed]
- Liu P, Li HF, Gao X, et al. The value of systematic clinical examinations in determining the involved muscles and clinical classification of myasthenia gravis patients. Chin J Neuroimmunol Neurol 2013;20:241-5.
- Skeie GO, Romi F. Paraneoplastic myasthenia gravis: immunological and clinical aspects. Eur J Neurol 2008;15:1029-33. [Crossref] [PubMed]
- Benatar M, Kaminski HJ, Quality Standards Subcommittee of the American Academy of Neurology. Evidence report: the medical treatment of ocular myasthenia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2007;68:2144-9. [Crossref] [PubMed]
- Skeie GO, Apostolski S, Evoli A, et al. European Federation of Neurological Societies. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol 2010;17:893-902. [Crossref] [PubMed]
- Kerty E, Elsais A, Argov Z, et al. EFNS/ENS Guidelines for the treatment of ocular myasthenia. Eur J Neurol 2014;21:687-93. [Crossref] [PubMed]
- Pirronti T, Rinaldi P, Batocchi AP, et al. Thymic lesions and myasthenia gravis. Diagnosis based on mediastinal imaging and pathological findings. Acta Radiol 2002;43:380-4. [Crossref] [PubMed]
- Tomiyama N, Honda O, Tsubamoto M, et al. Anterior mediastinal tumors: diagnostic accuracy of CT and MRI. Eur J Radiol 2009;69:280-8. [Crossref] [PubMed]
- Sugawara M, Wada C, Okawa S, et al. Long-term follow up of thymus in patients with myasthenia gravis. J Neuroimmunol 2010;221:121-4. [Crossref] [PubMed]
- Fiorelli A, Vicidomini G, Laperuta P, et al. The role of Tc-99m-2-Methoxy-Isobutyl-Isonitrile Single Photon Emission Computed Tomography in visualizing anterior mediastinal tumor and differentiating histologic type of thymoma. Eur J Cardiothorac Surg 2011;40:136-42. [Crossref] [PubMed]
- Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol 2009;8:475-90. [Crossref] [PubMed]
- Berrih-Aknin S, Frenkian-Cuvelier M, Eymard B. Diagnostic and clinical classification of autoimmune myasthenia gravis. J Autoimmun 2014;48-49:143-8. [Crossref] [PubMed]
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 2015;14:1023-36. [Crossref] [PubMed]
- Rodríguez Cruz PM, Al-Hajjar M, Huda S, et al. Clinical features and diagnostic usefulness of antibodies to clustered acetylcholine receptors in the diagnosis of seronegative myasthenia gravis. JAMA Neurol 2015;72:642-9. [Crossref] [PubMed]
- Vincent A, Newsom-Davis J. Acetylcholine receptor antibody as a diagnostic test for myasthenia gravis: results in 153 validated cases and 2967 diagnostic assays. J Neurol Neurosurg Psychiatry 1985;48:1246-52. [Crossref] [PubMed]
- Andrews PI, Massey JM, Sanders DB. Acetylcholine receptor antibodies in juvenile myasthenia gravis. Neurology 1993;43:977-82. [Crossref] [PubMed]
- Link H, Huang YM. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: an update on methodology and clinical usefulness. J Neuroimmunol 2006;180:17-28. [Crossref] [PubMed]
- Nowak RJ, Dicapua DB, Zebardast N, et al. Response of patients with refractory myasthenia gravis to rituximab: a retrospective study. Ther Adv Neurol Disord 2011;4:259-66. [Crossref] [PubMed]
- Drachman DB, Jones RJ, Brodsky RA. Treatment of refractory myasthenia: "rebooting" with high-dose cyclophosphamide. Ann Neurol 2003;53:29-34. [Crossref] [PubMed]
- Evoli A, Bianchi MR, Riso R, et al. Response to therapy in myasthenia gravis with anti-MuSK antibodies. Ann N Y Acad Sci 2008;1132:76-83. [Crossref] [PubMed]
- Yoshikawa H, Kiuchi T, Saida T, et al. Randomised, double-blind, placebo-controlled study of tacrolimus in myasthenia gravis. J Neurol Neurosurg Psychiatry 2011;82:970-7. [Crossref] [PubMed]
- Romi F, Gilhus NE, Varhaug JE, et al. Thymectomy in nonthymoma early-onset myasthenia gravis in correlation with disease severity and muscle autoantibodies. Eur Neurol 2003;49:210-7. [Crossref] [PubMed]
- Neves M, Alves JD. Factors implicated in the generation and persistence of long-lived plasma cell-mediated autoimmunity. Autoimmun Rev 2011;10:375-82. [Crossref] [PubMed]
- Osserman KE, Genkins G. Studies in myasthenia gravis: review of a twenty-year experience in over 1200 patients. Mt Sinai J Med 1971;38:497-537. [PubMed]
- Jaretzki A 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology 2000;55:16-23. [Crossref] [PubMed]
- Oosterhuis HJ. Observations of the natural history of myasthenia gravis and the effect of thymectomy. Ann N Y Acad Sci 1981;377:678-90. [Crossref] [PubMed]
- Mygland A, Aarli JA, Matre R, et al. Ryanodine receptor antibodies related to severity of thymoma associated myasthenia gravis. J Neurol Neurosurg Psychiatry 1994;57:843-6. [Crossref] [PubMed]
- Barohn RJ, McIntire D, Herbelin L, et al. Reliability testing of the quantitative myasthenia gravis score. Ann N Y Acad Sci 1998;841:769-772. [Crossref] [PubMed]
- Burns TM, Conaway MR, Sanders DB, et al. The MG composite: a valid and reliable outcome measure for myasthenia gravis. Neurology 2010;74:1434-1440. [Crossref] [PubMed]
- Gajdos P, Chevret S, Clair B, et al. Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. Myasthenia Gravis Clinical Study Group. Ann Neurol 1997;41:789-96. [Crossref] [PubMed]
- Wang XY, Xu XH, Sun H. A clinical absolute and relative score system for myasthenia gravis. Zhonghua Shen Jing Ke Za Zhi 1997;30:87-90.
- Gao X, Zhang X, Yang H, et al. Evaluation of the scales assessing the severity of myasthenia graves. Zhonghua Shen Jing Ke Za Zhi 2016. [Epub ahead of print].
- Smith AG, Burns TM. Reevaluating clinical measurement tools in therapeutic trials: time to make a Rasch decision? Neurology 2014;83:2104-5. [Crossref] [PubMed]
- van Nes SI, Faber CG, Merkies IS. Outcome measures in immune-mediated neuropathies: the need to standardize their use and to understand the clinimetric essentials. J Peripher Nerv Syst 2008;13:136-47. [Crossref] [PubMed]
- Sadjadi R, Conaway M, Cutter G, et al. Psychometric evaluation of the myasthenia gravis composite using Rasch analysis. Muscle Nerve 2012;45:820-5. [Crossref] [PubMed]
- Grob D, Brunner N, Namba T, et al. Lifetime course of myasthenia gravis. Muscle Nerve 2008;37:141-9. [Crossref] [PubMed]
- Li HF, Gao X, Xie YC. Recommendations for myasthenia gravis clinical trials. Muscle Nerve 2013;47:144-5. [Crossref] [PubMed]
- Mao ZF, Yang LX, Mo XA, et al. Frequency of autoimmune diseases in myasthenia gravis: a systematic review. Int J Neurosci 2011;121:121-9. [Crossref] [PubMed]
- Suzuki S, Utsugisawa K, Suzuki N. Overlooked non-motor symptoms in myasthenia gravis. J Neurol Neurosurg Psychiatry 2013;84:989-94. [Crossref] [PubMed]
- Sanders DB, Siddiqi ZA. Lessons from two trials of mycophenolate mofetil in myasthenia gravis. Ann N Y Acad Sci 2008;1132:249-53. [Crossref] [PubMed]
- Bedlack RS, Simel DL, Bosworth H, Samsa G, Tucker-Lipscomb B, Sanders DB. Quantitative myasthenia gravis score: assessment of responsiveness and longitudinal validity. Neurology 2005;64:1968-70. [Crossref] [PubMed]
- Barth D, Nabavi Nouri M, Ng E, et al. Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology 2011;76:2017-23. [Crossref] [PubMed]
- Katzberg HD, Barnett C, Merkies IS, et al. Minimal clinically important difference in myasthenia gravis: outcomes from a randomized trial. Muscle Nerve 2014;49:661-5. [Crossref] [PubMed]
- Quax RA, Koper JW, Huisman AM, et al. Polymorphisms in the glucocorticoid receptor gene and in the glucocorticoid-induced transcript 1 gene are associated with disease activity and response to glucocorticoid bridging therapy in rheumatoid arthritis. Rheumatol Int 2015;35:1325-33. [Crossref] [PubMed]
- O'Rourke K, Walsh C, Hutchinson M. Outcome of beta-interferon treatment in relapsing-remitting multiple sclerosis: a Bayesian analysis. J Neurol 2007;254:1547-54. [Crossref] [PubMed]
- Barnett C, Katzberg HD, Keshavjee S, et al. Thymectomy for non-thymomatous myasthenia gravis: a propensity score matched study. Orphanet J Rare Dis 2014;9:214. [Crossref] [PubMed]
- Roxburgh RH, Seaman SR, Masterman T, et al. Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology 2005;64:1144-51. [Crossref] [PubMed]
- Daumer M, Neuhaus A, Herbert J, et al. Prognosis of the individual course of disease: the elements of time, heterogeneity and precision. J Neurol Sci 2009;287 Suppl1:S50-5. [Crossref] [PubMed]
- Ohta K, Shigemoto K, Kubo S, et al. MuSK antibodies in AChR Ab-seropositive MG vs AChR Ab-seronegative MG. Neurology 2004;62:2132-3. [Crossref] [PubMed]
- Tsonis AI, Zisimopoulou P, Lazaridis K, et al. MuSK autoantibodies in myasthenia gravis detected by cell based assay--A multinational study. J Neuroimmunol 2015;284:10-7. [Crossref] [PubMed]
- Zisimopoulou P, Evangelakou P, Tzartos J, et al. A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J Autoimmun 2014;52:139-45. [Crossref] [PubMed]
- Della Marina A, Trippe H, Lutz S, et al. Juvenile myasthenia gravis: recommendations for diagnostic approaches and treatment. Neuropediatrics 2014;45:75-83. [Crossref] [PubMed]
- Han JL, Li HF, Xie YC, et al. Association between vitamin D receptor gene Tru9I polymorphism and myasthenia gravis. Zhonghua Yi Xue Za Zhi 2012;92:2028-33. [PubMed]
- Yue YX, Hong Y, Xie Y, et al. Association study between IL-17A and IL-17F gene polymorphism and myasthenia gravis in Chinese patients. Neurol Sci 2016;37:123-30. [Crossref] [PubMed]
- Akaishi T, Yamaguchi T, Suzuki Y, et al. Insights into the classification of myasthenia gravis. PLoS One 2014;9:e106757. [Crossref] [PubMed]
- Rao S, Olson JM, Moser KL, et al. Linkage analysis of human systemic lupus erythematosus-related traits: a principal component approach. Arthritis Rheum 2001;44:2807-18. [Crossref] [PubMed]
- Li PH, Wong WH, Lee TL, et al. Relationship between autoantibody clustering and clinical subsets in SLE: cluster and association analyses in Hong Kong Chinese. Rheumatology (Oxford) 2013;52:337-45. [Crossref] [PubMed]
- Morris DL, Vyse TJ. Analysis of systemic lupus erythematosus sub-phenotype data for genetic association. Curr Opin Rheumatol 2012;24:482-8. [Crossref] [PubMed]
- Oosterhuis HJ, Limburg PC, Hummel-Tappel E, The TH. Anti-acetylcholine receptor antibodies in myasthenia gravis. Part 2. Clinical and serological follow-up of individual patients. J Neurol Sci 1983;58:371-85. [Crossref] [PubMed]
- Heldal AT, Eide GE, Romi F, Owe JF, Gilhus NE. Repeated acetylcholine receptor antibody-concentrations and association to clinical myasthenia gravis development. PLoS One 2014;9:e114060. [Crossref] [PubMed]
- Huang DR, Pirskanen R, Matell G, et al. Tumour necrosis factor-alpha polymorphism and secretion in myasthenia gravis. J Neuroimmunol 1999;94:165-71. [Crossref] [PubMed]
- Dalakas MC. Future perspectives in target-specific immunotherapies of myasthenia gravis. Ther Adv Neurol Disord 2015;8:316-27. [Crossref] [PubMed]
- Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet 2012;379:1214-24. [Crossref] [PubMed]
- Hong Y, Hao HJ, Xie YC, et al. Effect of storage conditions and freeze/thaw cycles on serum and plasma levels of anti-acetylcholine receptor (AChR) antibody. Clin Chem Lab Med 2014;52:e103-5. [Crossref] [PubMed]
- Sulik A, Wojtkowska M, Oldak E. Preanalytical factors affecting the stability of matrix metalloproteinase-2 concentrations in cerebrospinal fluid. Clin Chim Acta 2008;392:73-5. [Crossref] [PubMed]
- Leung CH, Tseng HK, Wang WS, et al. Clinical characteristics of children and adults hospitalized for influenza virus infection. J Microbiol Immunol Infect 2014;47:518-25. [Crossref] [PubMed]
- Saver JL, Warach S, Janis S, et al. Standardizing the structure of stroke clinical and epidemiologic research data: the National Institute of Neurological Disorders and Stroke (NINDS) Stroke Common Data Element (CDE) project. Stroke 2012;43:967-73. [Crossref] [PubMed]
- Majersik JJ, Cole JW, Golledge J, et al. Recommendations from the international stroke genetics consortium, part 1: standardized phenotypic data collection. Stroke 2015;46:279-84. [Crossref] [PubMed]
- Baggi F, Mantegazza R, Antozzi C, et al. Patient registries: useful tools for clinical research in myasthenia gravis. Ann N Y Acad Sci 2012;1274:107-13. [Crossref] [PubMed]
- Fulvio B, Mantegazza R. European database for myasthenia gravis: a model for an international disease registry. Neurology 2014;83:189-91. [Crossref] [PubMed]
- Available online: www.commondataelements.ninds.nih.gov/MG.aspx
- Ng JK, Ng CS, Underwood MJ, et al. Does repeat thymectomy improve symptoms in patients with refractory myasthenia gravis? Interact Cardiovasc Thorac Surg 2014;18:376-80. [Crossref] [PubMed]
- Huang J, Detterbeck FC, Wang Z, et al. Standard outcome measures for thymic malignancies. J Thorac Oncol 2010;5:2017-23. [Crossref] [PubMed]
- Huang J, Ahmad U, Antonicelli A, et al. Development of the international thymic malignancy interest group international database: an unprecedented resource for the study of a rare group of tumors. J Thorac Oncol 2014;9:1573-8. [Crossref] [PubMed]
- Benveniste MF, Korst RJ, Rajan A, et al. A practical guide from the International Thymic Malignancy Interest Group (ITMIG) regarding the radiographic assessment of treatment response of thymic epithelial tumors using modified RECIST criteria. J Thorac Oncol 2014;9:S119-24. [Crossref] [PubMed]
- Detterbeck FC, Asamura H, Crowley J, et al. The IASLC/ITMIG thymic malignancies staging project: development of a stage classification for thymic malignancies. J Thorac Oncol 2013;8:1467-73. [Crossref] [PubMed]
- Bhora FY, Chen DJ, Detterbeck FC, et al. The ITMIG/IASLC Thymic Epithelial Tumors Staging Project: A Proposed Lymph Node Map for Thymic Epithelial Tumors in the Forthcoming 8th Edition of the TNM Classification of Malignant Tumors. J Thorac Oncol 2014;9:S88-96.
- Marx A, Strobel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol 2014;9:596-611. [Crossref] [PubMed]
- Teunissen C, Menge T, Altintas A, et al. Consensus definitions and application guidelines for control groups in cerebrospinal fluid biomarker studies in multiple sclerosis. Mult Scler 2013;19:1802-9. [Crossref] [PubMed]
- Little J, Higgins JP, Ioannidis JP, et al. STrengthening the REporting of Genetic Association studies (STREGA): an extension of the STROBE Statement. Ann Intern Med 2009;150:206-15. [Crossref] [PubMed]
- Gallo V, Egger M, McCormack V, et al. STrengthening the reporting of OBservational studies in epidemiology--molecular epidemiology STROBE-ME: an extension of the STROBE statement. J Clin Epidemiol 2011;64:1350-63. [Crossref] [PubMed]
- Worthy G. Statistical analysis and reporting: common errors found during peer review and how to avoid them. Swiss Med Wkly 2015;145:w14076. [PubMed]
- Nuzzo R. How scientists fool themselves - and how they can stop. Nature 2015;526:182-5. [Crossref] [PubMed]
- Hennekens CH, DeMets D. Statistical association and causation: contributions of different types of evidence. JAMA 2011;305:1134-5. [Crossref] [PubMed]


