
The prognostic and predictive value of mismatch repair status in patients with locally advanced rectal cancer following neoadjuvant therapy
Introduction
With 1.8 million new cases and 0.8 million deaths each year colorectal cancer (CRC) is the 3rd most commonly diagnosed cancer, and the 2nd cause of cancer-related death worldwide (1). CRC is a disease that develops via 2 well-described heterogeneous pathways of colorectal carcinogenesis; that is, chromosomal instability and, less commonly, microsatellite instability (MSI) (2,3). MSI is a consequence of a deficient mismatch repair (dMMR) system that results in the accumulation of insertion and/or deletion mutations within microsatellite deoxyribonucleic acid (DNA) regions (4,5). The MMR system is an evolutionarily high conserved system that recognizes mismatches and repairs DNA errors (6). Deficient MMR can result from the inheritance of a germline mutation in an MMR gene (e.g., MLH1, MSH2, MSH6, or PMS2), a situation called Lynch Syndrome, or more commonly, from the epigenetic inactivation of MLH1 in sporadic cases, often associated with the CpG island methylator phenotype (7,8). Thus, dMMR refers to a loss of function of MMR system, while proficient MMR (pMMR) refers to a proficient function of MMR system (6). Several studies and systematic reviews have shown that dMMR is associated with a more favorable stage-adjusted prognosis in non-metastatic colon cancer, and that 5-fluorouracil (5-FU) adjuvant therapy provides no benefits in stage II patients (9-16). Emerging evidence also suggests that dMMR can be predictive of a durable response and survival gain from immune checkpoint inhibitors (e.g., programmed cell death protein 1) in advanced and metastatic CRCs (17-19).
To date, the implications of MMR status have not been fully evaluated in relation to locally advanced rectal cancer for which dMMR prevalence has been reported to be <10% with a gradual decrease in its distribution from the proximal colon to the rectum (20,21). It remains unknown whether dMMR could predict tumor responses to neoadjuvant therapy, including chemoradiation or chemotherapy alone, or whether dMMR could be used as a prognostic marker for oncological outcomes after neoadjuvant therapy. Li et al. reported the predictive value of MMR in gastric and gastroesophageal junction adenocarcinoma patients receiving neoadjuvant chemotherapy. No significant difference was found in the terms of tumor regression grade (TRG) between pMMR and dMMR tumors (22). However, the predictive value of MMR status in rectal cancer receiving neoadjuvant therapy was unknown. Thus, the present study sought to investigate the predictive and prognostic value of dMMR in locally advanced rectal cancer patients who had undergone neoadjuvant therapy including chemotherapy alone and chemoradiation therapy. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-124/rc)
Methods
Study population
This study was performed in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of the Sixth Affiliated Hospital, Sun Yat-sen University (No. 2022ZSLYEC-091) and individual consent for this retrospective analysis was waived.
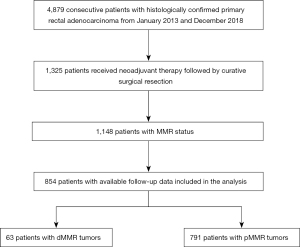
This retrospective study included all consecutive patients with histologically confirmed locally advanced rectal cancer and MMR status who underwent neoadjuvant therapy followed by curative surgical resection from January 2013 to December 2018 at The Sixth Affiliated Hospital of Sun Yat-sen University. Patients in the last three years were not included due to immature survival data. Patients with synchronous multiple primary cancer, inflammatory bowel disease, or familial adenomatous polyposis were excluded from the study. The selection process for this study is outlined in Figure 1.
IHC analysis of MMR expression
MLH1, MSH2, MSH6, and PMS2 proteins were stained by immunohistochemistry (IHC), with formalin-fixed, paraffin-embedded tumors. Negative nuclear staining in neoplastic cells, with positive nuclear staining in lymphocytes and normal adjacent colonic epithelium, were defined as MMR loss (23). Primary monoclonal antibodies against MLH1 (clone ES05; Zhong Shan Jin Qiao, Beijing, China), MSH2 (clone RED2; Zhong Shan Jin Qiao, Beijing, China), MSH6 (clone UMAB258; Zhong Shan Jin Qiao, Beijing, China), and PMS2 (clone EP51; Zhong Shan Jin Qiao, Beijing, China) were applied. Representative images of IHC were provided in Figure S1.
MMR status determination
MMR status was determined by a detection of MMR protein expression by IHC, and MSI testing by polymerase chain reaction (PCR) was used if the result of IHC was uncertain. Deficient MMR phenotype tumors were defined as tumors with 1 or more loss expression of MMR proteins by IHC (23). Tumors with discordant results of MMR protein and DNA MSI testing were excluded from this study.
Treatment and follow-up
All patients received surgery with total mesorectal excision after neoadjuvant therapy, including infusional fluorouracil (the de Gramont regimen) or mFOLFOX6 plus radiotherapy, and neoadjuvant chemotherapy with mFOLFOX6 or the mFOLFOXIRI regimen alone. Radiotherapy was administered at 2.0 Gy for 25 fractions over 5 weeks, with a total dose of 50 Gy (24-26). A physical examination, serum carcinoembryonic antigen test, and computed tomography scan (chest/abdominal/pelvic) with a frequency of every 3–6 months for the first 3 years after surgery and then every 6 months for the following 2 years, were the routine follow-up strategy for all patients. The data were updated in August 2019.
Propensity score matching
Propensity score matching was performed to reduce bias of the baseline characteristics between patients with different MMR statuses in the chemotherapy and chemoradiation groups. A multivariable logistic regression model was constructed to generate propensity scores. Factors presumed to be associated with the patients’ tumor responses after neoadjuvant therapy were selected in the propensity model. The following baseline data were included in the model: ≥65 years, sex, grade of differentiation, mucus, low location, clinical tumor (T) stage, and clinical node (N) stage. Patients with dMMR were matched to those with pMMR at a 1:4 ratio using a greedy nearest-neighbor matching algorithm with no replacement. Baseline characteristics were compared between the propensity score-matched group using standardized mean differences (SMDs). A SMD <0.1 indicated a negligible imbalance between groups (27).
Statistical analysis
The primary endpoint was the effect of dMMR on tumor response to neoadjuvant therapy in the overall cohort and the different neoadjuvant patterns. TRG was evaluated semi-quantitatively on a scale of 0 to 3 (complete to poor response, respectively) according to the American Joint Committee on Cancer system. Pathologic complete response was defined as the absence of viable tumor cells in the surgical specimens, including in the primary tumor area, whole mesorectal fat, and the resected lymph nodes (ypT0N0).
The 2nd endpoint was the relationship between MMR status and Local recurrence-free survival (LRFS) and disease-free survival (DFS) in the overall cohort. LRFS was defined as the time from surgery to tumor regrowth within the pelvis or perineum. DFS was defined as the time from surgery to the first event of local or metastatic recurrence, second primary cancer, or death from any cause.
The categorical variables were compared using the Chi-square test or Fisher’s exact test. LRFS and DFS curves were estimated using the Kaplan-Meier method, and were compared using a Cox proportional hazards regression model with HRs, 95% CI, and P values for the candidate prognostic factors. Variables with P values <0.05 in the univariate analysis or considered clinically significant were included in the multivariate analysis. Two-sided P values <0.05 were considered statistically significant. All the statistical analyses were performed with SPSS software (version 22, SPSS Inc., Chicago, IL, USA), except that the propensity score matching was implemented in R, version 3.3.2 (R Foundation), using the package MatchIt.
Results
Patient characteristics
A total of 854 patients with clinical stage II (23.4%) or III (76.6%) disease at the time of diagnosis were enrolled in this study. The patients had a median age of 55 years (range, 19–80 years) at diagnosis, and 71.5% were male. Among the 854 patients tested for MMR status by IHC, 63 patients (7.4%) had dMMR tumors. No significant differences were observed between the baseline characteristics and MMR status, except that dMMR patients were more likely to be younger at the time of diagnosis (<65 years, 88.9% vs. 77.7%; P=0.056) and have mucinous adenocarcinoma (12.7% vs. 4.4%; P=0.010; Table 1).
Table 1
| Variables | Total (n=854), n (%) | dMMR (n=63), n (%) | pMMR (n=791), n (%) | P |
|---|---|---|---|---|
| Age, years | 0.056 | |||
| <65 | 671 (78.6) | 56 (88.9) | 615 (77.7) | |
| ≥65 | 183 (21.4) | 7 (11.1) | 176 (22.3) | |
| Gender | 0.679 | |||
| Male | 611 (71.5) | 47 (74.6) | 564 (71.3) | |
| Female | 243 (28.5) | 16 (25.4) | 227 (28.7) | |
| Grade of differentiation | 0.223 | |||
| Well/moderate | 764 (89.5) | 53 (84.1) | 711 (89.9) | |
| Poor | 90 (10.5) | 10 (15.9) | 80 (10.1) | |
| Mucinous adenocarcinoma | 0.010 | |||
| No | 811 (95.0) | 55 (87.3) | 756 (95.6) | |
| Yes | 43 (5.0) | 8 (12.7) | 35 (4.4) | |
| Location from anal verge, cm | 0.153 | |||
| <5 | 411 (48.1) | 27 (42.9) | 384 (48.5) | |
| 5–10 | 376 (44.0) | 27 (42.9) | 349 (44.1) | |
| >10 | 67 (7.8) | 9 (14.3) | 58 (7.3) | |
| Clinical T stage | 0.124 | |||
| T2 | 15 (1.8) | 2 (3.2) | 13 (1.6) | |
| T3 | 659 (77.2) | 43 (68.3) | 616 (77.9) | |
| T4 | 180 (21.1) | 18 (28.6) | 162 (20.5) | |
| Clinical N stage | 0.396 | |||
| N0 | 200 (23.4) | 18 (28.6) | 182 (23.0) | |
| N1–2 | 654 (76.6) | 45 (71.4) | 609 (77.0) |
dMMR, deficient mismatch repair; pMMR, proficient mismatch repair.
Tumor responses to neoadjuvant therapy according to MMR status
The associations between MMR status and the postoperative pathological characteristics that reflect tumor responses to neoadjuvant therapy are listed in Table 2. Among the 854 enrolled patients, 420 (49.2%) received neoadjuvant fluorouracil-based chemotherapy, consisting of mFOLFOX6 (n=264, 30.9%) or the De Gramont regimen (n=156, 18.3%), concurrently with long-course pelvic radiation, and 434 (50.8%) received neoadjuvant chemotherapy alone. The neoadjuvant therapy regimens were generally well balanced between patients with dMMR and pMMR status. After neoadjuvant therapy, patients with dMMR had a lower proportion of TRG of 0–1 compared with pMMR patients (28.6% vs. 43.7%; P=0.027). However, no significant association was observed between MMR status and neoadjuvant therapy efficacy with respect to ypT, ypN, and ypTNM stages, and pathological complete response (pCR). 15.9%, 28.6%, 38.1%, and 17.5% of dMMR patients, and 12.9%, 30.6%, 34.5%, and 22.0%, of pMMR patients had ypTNM stage T0N0, I, II, and III, respectively. pCR was achieved in 112 cases (13.1%), at a rate of 15.9% in dMMR patients and 12.9% in pMMR patients (Table 2).
Table 2
| Variables | Total (n=854), n (%) | dMMR (n=63), n (%) | pMMR (n=791), n (%) | P |
|---|---|---|---|---|
| Neoadjuvant therapy | 0.878 | |||
| Infusional fluorouracil plus radiotherapy | 156 (18.3) | 10 (15.9) | 146 (18.5) | |
| mFOLFOX6 plus radiotherapy | 264 (30.9) | 20 (31.7) | 244 (30.8) | |
| Neoadjuvant chemotherapy alone | 434 (50.8) | 33 (52.4) | 401 (50.7) | |
| ypT stage | 0.686 | |||
| T0 | 123 (14.4) | 11 (17.5) | 112 (14.2) | |
| T1 | 74 (8.7) | 6 (9.5) | 68 (8.6) | |
| T2 | 210 (24.6) | 12 (19.0) | 198 (25.0) | |
| T3 | 419 (49.1) | 31 (49.2) | 388 (49.1) | |
| T4 | 28 (3.3) | 3 (4.8) | 25 (3.2) | |
| ypN stage | 0.088 | |||
| N0 | 669 (78.3) | 52 (82.5) | 617 (78.0) | |
| N1 | 137 (16.0) | 11 (17.5) | 126 (15.9) | |
| N2 | 48 (5.6) | 0 (0.0) | 48 (6.1) | |
| ypTNM stage | 0.743 | |||
| T0N0 | 112 (13.1) | 10 (15.9) | 102 (12.9) | |
| Stage I | 260 (30.4) | 18 (28.6) | 242 (30.6) | |
| Stage II | 297 (34.8) | 24 (38.1) | 273 (34.5) | |
| Stage III | 185 (21.7) | 11 (17.5) | 174 (22.0) | |
| pCR | 0.631 | |||
| No | 742 (86.9) | 53 (84.1) | 689 (87.1) | |
| Yes | 112 (13.1) | 10 (15.9) | 102 (12.9) | |
| TRG | 0.027 | |||
| 0–1 | 364 (42.6) | 18 (28.6) | 346 (43.7) | |
| 2–3 | 490 (57.4) | 45 (71.4) | 445 (56.3) | |
| Postoperative chemotherapy | 0.480 | |||
| Untreated | 118 (13.8) | 8 (12.7) | 110 (13.9) | |
| Fluoropyrimidine-based | 122 (14.3) | 6 (9.5) | 116 (14.7) | |
| Oxaliplatin-based | 614 (71.9) | 49 (77.8) | 565 (71.4) |
dMMR, deficient mismatch repair; pMMR, proficient mismatch repair; pCR, pathologic complete response; TRG, tumor regression grade.
Tumor responses to chemotherapy alone according to MMR status
For patients who received chemotherapy alone, after 1:4 propensity score matching, 33 patients with dMMR were matched to 132 patients with pMMR. After propensity score matching, the SMDs for most of the included covariates among patients with dMMR and pMMR were <0.1, indicating a well-balanced covariate distribution (Table S1). After matching, the proportion of TRG 0–1 was obviously lower in patients with dMMR (9.1% vs. 30.3%; P=0.013). However, the pCR rates were similar between the dMMR and pMMR groups (6.1% vs. 6.1%; Table 3).
Table 3
| Variables | Total (n=165), n (%) | dMMR (n=33), n (%) | pMMR (n=132), n (%) | P |
|---|---|---|---|---|
| ypT stage | 0.760 | |||
| T0 | 12 (7.3) | 2 (6.1) | 10 (7.6) | |
| T1 | 11 (6.7) | 3 (9.1) | 8 (6.1) | |
| T2 | 38 (23.0) | 9 (27.3) | 29 (22.0) | |
| T3 | 98 (59.4) | 17 (51.5) | 81 (61.4) | |
| T4 | 6 (3.6) | 2 (6.1) | 4 (3.0) | |
| ypN stage | 0.187 | |||
| N0 | 122 (73.9) | 27 (81.8) | 95 (72.0) | |
| N1 | 31 (18.8) | 6 (18.2) | 25 (18.9) | |
| N2 | 12 (7.3) | 0 (0.0) | 12 (9.1) | |
| ypTNM stage | 0.562 | |||
| T0N0 | 10 (6.1) | 2 (6.1) | 8 (6.1) | |
| Stage I | 46 (27.9) | 12 (36.4) | 34 (25.8) | |
| Stage II | 66 (40.0) | 13 (39.4) | 53 (40.2) | |
| Stage III | 43 (26.1) | 6 (18.2) | 37 (28.0) | |
| pCR | 1.000 | |||
| No | 155 (93.9) | 31 (93.9) | 124 (93.9) | |
| Yes | 10 (6.1) | 2 (6.1) | 8 (6.1) | |
| TRG | 0.013 | |||
| 0–1 | 43 (26.1) | 3 (9.1) | 40 (30.3) | |
| 2–3 | 122 (73.9) | 30 (90.9) | 92 (69.7) | |
| Postoperative chemotherapy | 1.000 | |||
| Untreated | 16 (9.7) | 3 (9.1) | 13 (9.8) | |
| Fluoropyrimidine-based | 3 (1.8) | 0 (0.0) | 3 (2.3) | |
| Oxaliplatin-based | 146 (88.5) | 30 (90.9) | 116 (87.9) |
dMMR, deficient mismatch repair; pMMR, proficient mismatch repair; pCR, pathologic complete response; TRG, tumor regression grade.
Tumor responses to chemoradiation therapy according to MMR status
For patients who received chemoradiation, at 1:4 propensity score matching, 30 patients with dMMR were matched to 120 patients with pMMR. The SMDs of the included covariates are shown in Table S2. After matching, no significant difference in the proportion of TRG 0–1 and pCR rate was observed between patients with dMMR and pMMR (50.0% vs. 64.2%; P=0.224 and 26.7% vs. 22.5%; P=0.809; Table S2 and Table 4).
Table 4
| Variables | Total (n=150), n (%) | dMMR (n=30), n (%) | pMMR (n=120), n (%) | P |
|---|---|---|---|---|
| ypT stage | 0.257 | |||
| T0 | 39 (26.0) | 9 (30.0) | 30 (25.0) | |
| T1 | 14 (9.3) | 3 (10.0) | 11 (9.2) | |
| T2 | 36 (24.0) | 3 (10.0) | 33 (27.5) | |
| T3 | 58 (38.7) | 14 (46.7) | 44 (36.7) | |
| T4 | 3 (2.0) | 1 (3.3) | 2 (1.7) | |
| ypN stage | 0.666 | |||
| N0 | 124 (82.7) | 25 (83.3) | 99 (82.5) | |
| N1 | 21 (14.0) | 5 (16.7) | 16 (13.3) | |
| N2 | 5 (3.3) | 0 (0.0) | 5 (4.2) | |
| ypTNM stage | 0.486 | |||
| T0N0 | 35 (23.3) | 8 (26.7) | 27 (22.5) | |
| Stage I | 45 (30.0) | 6 (20.0) | 39 (32.5) | |
| Stage II | 44 (29.3) | 11 (36.7) | 33 (27.5) | |
| Stage III | 26 (17.3) | 5 (16.7) | 21 (17.5) | |
| pCR | 0.809 | |||
| No | 115 (76.7) | 22 (73.3) | 93 (77.5) | |
| Yes | 35 (23.3) | 8 (26.7) | 27 (22.5) | |
| TRG | 0.224 | |||
| 0–1 | 92 (61.3) | 15 (50.0) | 77 (64.2) | |
| 2–3 | 58 (38.7) | 15 (50.0) | 43 (35.8) | |
| Postoperative chemotherapy | 0.468 | |||
| Untreated | 33 (22.0) | 5 (16.7) | 28 (23.3) | |
| Fluoropyrimidine-based | 37 (24.7) | 6 (20.0) | 31 (25.8) | |
| Oxaliplatin-based | 80 (53.3) | 19 (63.3) | 61 (50.8) |
dMMR, deficient mismatch repair; pMMR, proficient mismatch repair; pCR, pathologic complete response; TRG, tumor regression grade.
Survival according to MMR status
With an overall median follow-up period of 37.6 months, the 3-year local recurrence rates were 5.7% and 7.8% in the dMMR and pMMR patients, respectively. In the univariate analysis, MMR status was not significantly associated with LRFS (HR =0.83, 95% CI: 0.26–2.63; P=0.746; Figure 2A). In the multivariate analysis, which was adjusted for age, gender, grade of differentiation, MMR status, clinical N stage, neoadjuvant therapy pattern, pCR, TRG, ypT, and adjuvant chemotherapy, patients with a higher clinical T stage and ypN stage had significantly shorter LRFS (Table S3).
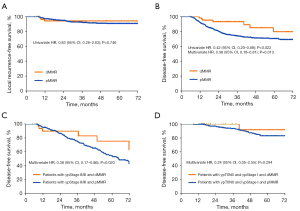
The 3-year DFS rates were 93.2% in patients with dMMR, and 73.9% in patients with pMMR. In the multivariate analysis, which was adjusted for age, sex, grade of differentiation, clinical T stage, clinical N stage, neoadjuvant therapy pattern, postoperative chemotherapy, ypT stage, ypN stage, pCR, and TRG, dMMR status was independently and significantly associated with longer DFS than pMMR status (HR =0.38, 95% CI: 0.18–0.81; P=0.013; Figure 2B, Table S3). In the subgroup multivariate analysis, dMMR status was only significantly associated with a longer DFS than pMMR status in patients with ypStage II/III disease (HR =0.38, 95% CI: 0.17–0.86; P=0.020); no such association was found in patients with ypT0N0 and Stage I disease (HR =0.34, 95% CI: 0.05–2.55; P=0.294; Table S4, Figure 2C,2D).
Survival according to neoadjuvant pattern and MMR status
Somewhat surprisingly, in the after-matched cohort of neoadjuvant chemotherapy and neoadjuvant chemoradiation, MMR status was not significantly associated with DFS in the univariate and multivariate analyses (Table S5). This may have been due to the limited number of patients.
Discussion
To our knowledge, our current study is the largest sample-sized analysis to examine the predictive and prognostic value of MMR status in patients with locally advanced rectal cancer following neoadjuvant therapy. Neoadjuvant therapy is recommended for patients with clinical stage II/III rectal cancer (28). However, few studies have evaluated the effect of neoadjuvant therapy patterns in dMMR patients with rectal cancer. Our findings indicate that patients with pMMR respond better to neoadjuvant therapy in terms of TRG than patients with dMMR. However, after matching, no difference in relation to TRG between dMMR and pMMR tumors was observed in the subgroup of patients who underwent neoadjuvant chemoradiotherapy, but patients with dMMR had a worse response to chemotherapy alone in terms of TRG than patients with pMMR.
Five previous retrospective studies have evaluated the effect of MMR status in rectal cancer patients treated with neoadjuvant therapy, but conflicting results have been reported. Meillan et al. reported on a series of 296 locally advanced rectal cancer patients who received chemoradiation, 23 of whom had dMMR status. They found that dMMR was associated with a higher pathologic downstaging rate but worse TRG (29). One problem of downstaging is that clinical staging is highly variable, as the different imaging modalities are not always accurate due to the large differences between clinical and pathological stages (30). The pCR rate, which is a potential surrogate for longer-term outcomes, was not reported, as all patients with pCR were excluded from this study. In an analysis of 636 MSI (+) patients from the National Cancer Database, MSI (+) patients were reported to have a more reduced pCR rate than MSI (–) patients (5.9% vs. 8.9%; P=0.01) (31). Conversely, 3 previous studies reported results similar to those of our study, and found no significant difference in the pCR rates between patients with dMMR/MSI and pMMR/MSS (32-34).
Data on neoadjuvant chemotherapy in CRC are scarce. In a phase III FOXTROT trial, which evaluated the efficacy of neoadjuvant chemotherapy in treating locally advanced colon cancer, 95% of the 106 patients with dMMR tumors who received neoadjuvant chemotherapy showed little or no response (35). Another retrospective study at Memorial Sloan Kettering included 21 dMMR patients who received neoadjuvant chemotherapy (fluorouracil/oxaliplatin). Of these 21 patients, 6 (29%) had progression of disease, compared to no progression in the matched 63 pMMR rectal tumors (36). Our data showed that in rectal cancer patients who received neoadjuvant chemotherapy, pMMR patients had a better response. Thus, chemotherapy alone should be administered with caution to dMMR patients who have received neoadjuvant therapy, especially when the tumor volume is large and significant tumor regression is required. One possible explanation for dMMR resistance to fluorouracil-based chemotherapy is that, in the absence of a functional MMR system, repair may only occur through the “base excision repair” system, a process that is less affected by the disequilibrium disequilibrium induced by 5-FU (37).
Despite dMMR patients having a poorer response to neoadjuvant chemotherapy than pMMR patients, the DFS of dMMR patients was not inferior in our study after propensity score matching. This might be due to the small number of dMMR patients, and thus the poor statistical power of this study. Alternately, this might be due to the good prognosis of patients with dMMR, and a poor response to neoadjuvant therapy may not have a significant effect on survival. Du et al. found that patients with MSI-H tumors had significantly better DFS than those with MSI-L and MSS tumors in a ypN0 subgroup (34). Conversely, we found that dMMR was a significantly good prognostic marker for DFS in patients with ypStage II/III, and observed a non-significant trend toward better DFS in dMMR patients in the ypT0N0/stage I subgroup. These results might be explained by the good prognosis of all patients with ypT0N0/stage I; however, the small sample size may not have been powerful enough to reveal a statistical difference between the dMMR and pMMR tumors.
Several previous studies have investigated the prognostic effect of MMR status in patients with rectal cancer following upfront surgery. Colombino et al. demonstrated that patients with MSI-H rectal cancers had better DFS and overall survival than those with MSI-L/MSS (38), but others have found no significant survival advantages in patients with MSI/dMMR (39-41). These results need to be interpreted with caution because of the small number (range, 12 to 24) of MSI/dMMR patients.
The main limitation of our study is that it was a retrospective study. Thus, selection bias cannot be excluded. The decision to administer neoadjuvant therapy was left to the investigators’ discretion after discussion with a multidisciplinary team, which was mainly based on the estimated risk of recurrence, age, and each patient's physical condition and preferences; however, we used propensity score matching to reduce the imbalance. The other potential predictive or prognostic molecular markers, such as RAS and BRAF, were absent. A pooled analysis of resected stage III colon cancer patients suggested BRAF or KRAS mutations are independently associated with a shorter time to recurrence in patients with MSS but not MSI tumors (42). All the patients in this study were determined to have MMR status by IHC. IHC with antibodies directed against MLH1, MSH2, MSH6, and PMS2 is the preferred approach in daily clinical practice due to its availability and costs. A review of 16 studies of 3,494 cases demonstrated that the sensitivity and specificity of IHC was essentially concordant with PCR-based MSI testing (43). However, a few cases of MSI with rare missense mutations cannot be detected by IHC, which is likely due to the retained antigenicity in an otherwise non-functional protein (44,45). In these cases, MSI testing by PCR can help to determine whether there are true functional MMR proteins.
In conclusion, we demonstrated that in terms of TRG, the response to neoadjuvant chemotherapy of patients with dMMR locally advanced rectal cancer was worse than that of pMMR patients. Also, dMMR is a significantly good prognostic marker for DFS in patients with ypStage II/III after neoadjuvant therapy.
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of Guangdong Province of China (grant No. 2019A1515010071).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-124/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-124/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-124/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ionov Y, Peinado MA, Malkhosyan S, et al. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993;363:558-61. [Crossref] [PubMed]
- Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology 2008;135:1079-99. [Crossref] [PubMed]
- Duval A, Hamelin R. Mutations at coding repeat sequences in mismatch repair-deficient human cancers: toward a new concept of target genes for instability. Cancer Res 2002;62:2447-54. [PubMed]
- Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 2006;7:335-46. [Crossref] [PubMed]
- Olave MC, Graham RP. Mismatch repair deficiency: The what, how and why it is important. Genes Chromosomes Cancer 2021; Epub ahead of print. [Crossref] [PubMed]
- Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature 2001;411:366-74. [Crossref] [PubMed]
- Ogino S, Goel A. Molecular classification and correlates in colorectal cancer. J Mol Diagn 2008;10:13-27. [Crossref] [PubMed]
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247-57. [Crossref] [PubMed]
- Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219-26. [Crossref] [PubMed]
- Benatti P, Gafà R, Barana D, et al. Microsatellite instability and colorectal cancer prognosis. Clin Cancer Res 2005;11:8332-40. [Crossref] [PubMed]
- Jover R, Zapater P, Castells A, et al. The efficacy of adjuvant chemotherapy with 5-fluorouracil in colorectal cancer depends on the mismatch repair status. Eur J Cancer 2009;45:365-73. [Crossref] [PubMed]
- Carethers JM, Smith EJ, Behling CA, et al. Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterology 2004;126:394-401. [Crossref] [PubMed]
- Jover R, Zapater P, Castells A, et al. Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer. Gut 2006;55:848-55. [Crossref] [PubMed]
- Des Guetz G, Schischmanoff O, Nicolas P, et al. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis. Eur J Cancer 2009;45:1890-6. [Crossref] [PubMed]
- Fotheringham S, Mozolowski GA, Murray EMA, et al. Challenges and solutions in patient treatment strategies for stage II colon cancer. Gastroenterol Rep (Oxf) 2019;7:151-61. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182-91. [Crossref] [PubMed]
- Le DT, Kim TW, Van Cutsem E, et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J Clin Oncol 2020;38:11-9. [Crossref] [PubMed]
- Gelsomino F, Barbolini M, Spallanzani A, et al. The evolving role of microsatellite instability in colorectal cancer: A review. Cancer Treat Rev 2016;51:19-26. [Crossref] [PubMed]
- Ryan E, Sheahan K, Creavin B, et al. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit Rev Oncol Hematol 2017;116:38-57. [Crossref] [PubMed]
- Li Z, Wang Y, Ying X, et al. Prognostic and predictive value of mismatch repair deficiency in gastric and gastroesophageal junction adenocarcinoma patients receiving neoadjuvant or adjuvant chemotherapy. J Surg Oncol 2021;124:1356-64. [Crossref] [PubMed]
- Luchini C, Bibeau F, Ligtenberg MJL, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol 2019;30:1232-43. [Crossref] [PubMed]
- Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 With or Without Radiation Versus Fluorouracil and Leucovorin With Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J Clin Oncol 2016;34:3300-7. [Crossref] [PubMed]
- Deng Y, Chi P, Lan P, et al. Neoadjuvant Modified FOLFOX6 With or Without Radiation Versus Fluorouracil Plus Radiation for Locally Advanced Rectal Cancer: Final Results of the Chinese FOWARC Trial. J Clin Oncol 2019;37:3223-33. [Crossref] [PubMed]
- Zhang J, Huang M, Cai Y, et al. Neoadjuvant Chemotherapy With mFOLFOXIRI Without Routine Use of Radiotherapy for Locally Advanced Rectal Cancer. Clin Colorectal Cancer 2019;18:238-44. [Crossref] [PubMed]
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. [Crossref] [PubMed]
- Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv22-40. [Crossref] [PubMed]
- Meillan N, Vernerey D, Lefèvre JH, et al. Mismatch Repair System Deficiency Is Associated With Response to Neoadjuvant Chemoradiation in Locally Advanced Rectal Cancer. Int J Radiat Oncol Biol Phys 2019;105:824-33. [Crossref] [PubMed]
- Nagtegaal ID, Glynne-Jones R. How to measure tumour response in rectal cancer? An explanation of discrepancies and suggestions for improvement. Cancer Treat Rev 2020;84:101964. [Crossref] [PubMed]
- Hasan S, Renz P, Wegner RE, et al. Microsatellite Instability (MSI) as an Independent Predictor of Pathologic Complete Response (PCR) in Locally Advanced Rectal Cancer: A National Cancer Database (NCDB) Analysis. Ann Surg 2020;271:716-23. [Crossref] [PubMed]
- Charara M, Edmonston TB, Burkholder S, et al. Microsatellite status and cell cycle associated markers in rectal cancer patients undergoing a combined regimen of 5-FU and CPT-11 chemotherapy and radiotherapy. Anticancer Res 2004;24:3161-7. [PubMed]
- Bertolini F, Bengala C, Losi L, et al. Prognostic and predictive value of baseline and posttreatment molecular marker expression in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys 2007;68:1455-61. [Crossref] [PubMed]
- Du C, Zhao J, Xue W, et al. Prognostic value of microsatellite instability in sporadic locally advanced rectal cancer following neoadjuvant radiotherapy. Histopathology 2013;62:723-30. [Crossref] [PubMed]
- Morton D. FOxTROT: An international randomised controlled trial in 1053 patients evaluating neoadjuvant chemotherapy (NAC) for colon cancer. On behalf of the FOxTROT Collaborative Group. Ann Oncol 2019;30:v198. [Crossref]
- Cercek A, Dos Santos Fernandes G, Roxburgh CS, et al. Mismatch Repair-Deficient Rectal Cancer and Resistance to Neoadjuvant Chemotherapy. Clin Cancer Res 2020;26:3271-9. [Crossref] [PubMed]
- Fischer F, Baerenfaller K, Jiricny J. 5-Fluorouracil is efficiently removed from DNA by the base excision and mismatch repair systems. Gastroenterology 2007;133:1858-68. [Crossref] [PubMed]
- Colombino M, Cossu A, Manca A, et al. Prevalence and prognostic role of microsatellite instability in patients with rectal carcinoma. Ann Oncol 2002;13:1447-53. [Crossref] [PubMed]
- Meng WJ, Sun XF, Tian C, et al. Microsatellite instability did not predict individual survival in sporadic stage II and III rectal cancer patients. Oncology 2007;72:82-8. [Crossref] [PubMed]
- Oh CR, Kim JE, Kang J, et al. Prognostic Value of the Microsatellite Instability Status in Patients With Stage II/III Rectal Cancer Following Upfront Surgery. Clin Colorectal Cancer 2018;17:e679-85. [Crossref] [PubMed]
- Hong SP, Min BS, Kim TI, et al. The differential impact of microsatellite instability as a marker of prognosis and tumour response between colon cancer and rectal cancer. Eur J Cancer 2012;48:1235-43. [Crossref] [PubMed]
- Taieb J, Le Malicot K, Shi Q, et al. Prognostic Value of BRAF and KRAS Mutations in MSI and MSS Stage III Colon Cancer. J Natl Cancer Inst 2016;109:djw272. [Crossref] [PubMed]
- Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn 2008;10:293-300. [Crossref] [PubMed]
- Klarskov L, Holck S, Bernstein I, et al. Challenges in the identification of MSH6-associated colorectal cancer: rectal location, less typical histology, and a subset with retained mismatch repair function. Am J Surg Pathol 2011;35:1391-9. [Crossref] [PubMed]
- Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol 2002;20:1043-8. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


