
Sodium-glucose co-transporter 1 (SGLT1) differentially regulates gluconeogenesis and GLP-1 receptor (GLP-1R) expression in different diabetic rats: a preliminary validation of the hypothesis of “SGLT1 bridge” as an indication for “surgical diabetes”
Introduction
For decades, the entero-insular axis (EIA) hypothesis has been the most popular and acceptable theory for elucidating the mechanisms of diabetes remission after metabolic and bariatric surgery (MBS). The Roux-en-Y gastric bypass (RYGB) is superior to sleeve gastrectomy (SG) for achieving diabetes remission (1) because more EIA-related hormones, known as incretin, are changed by bypass surgery. Glucagon-like peptide-1 (GLP-1) is considered one of the most important factors in the EIA hypothesis (2) and GLP-1 analogues and its receptor (GLP-1R) agonist are used for antidiabetic therapy (3). However, some studies (4-11) have expressed doubt over the role of GLP-1 in the context of the EIA hypothesis. Firstly, GLP-1 as a clinical antidiabetic drug has far fewer therapeutic effects than MBS. Secondly, the amount of GLP-1 secretion is not correlated with diabetes remission (8), and both increased (12) and decreased (13) GLP-1 secretion have been found after surgery. Further, in GLP-1R knockout mice, RYGB still exhibited improved glucose homeostasis (9,14), and a GLP-1R antagonist did not deteriorate glucose homeostasis in patients who achieved diabetes remission following RYGB (5). Our previous study (15) involving ileal transposition performed on Goto-Kakizaki (GK) rats showed rapid improvement of glucose tolerance and a delayed improvement of insulin resistance, accompanied with a decreased insulin level instead of the increased insulin secretion subsequently induced by increased GLP-1 expression (16,17), suggesting that it might have been glucose tolerance improvement rather than increased GLP-1 expression that led to the improvement of insulin resistance. Taken together, the EIA hypothesis cannot fully explain diabetic improvement in MBS, and further studies are required to define the role of GLP-1 in glucose metabolism (18).
Since the phenomenon cannot be well explained by the EIA hypothesis, the gut-brain-liver axis (GBLA) hypothesis has been raised as an alternative theory for elucidating the mechanisms of diabetes remission after MBS (10,19). Glucose in blood initiates a signal to the portal vein in fasting animals. The portal glucose signal (PGS) interferes with glucose homeostasis by regulating the production of glucose by the liver [hepatic gluconeogenesis (HGNG)] (20-22), transmitted by vagal afferents to the energy homeostasis center (the hypothalamus) and via the efferent nervous system to the liver. Ideally located just upstream of the PGS, intestinal gluconeogenesis (IGNG), which enhances the gluconeogenic effect by up to 20% during prolonged hunger (19), controls the intestinal glucose release into the portal vein to activate the PGS. In normal situations, during the postabsorptive period, the gluconeogenic function is expressed in the proximal intestine (23-25), whereas after gastric bypass, high expression of IGNG regulatory genes glucose-6 phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PCK) occurs in the distal small intestine (10). The increased G6pase and PCK expression and activity can be seen in the duodenum and alimentary limb after RYGB (26,27). Thus, it has been suggested (10,28,29) that the induction of IGNG plays a major role in endogenous glucose production (EGP), and the intact GBLA axis is the mechanical link key to rapid glucose improvement after gastric bypass.
Sodium-glucose co-transporter 1 (SGLT1), mainly located in the intestine, predominantly mediates glucose across the intestinal brush-border membrane (BBM) (30-32). The expression of SGLT1 in diabetic humans is 2–3 folds higher than in nondiabetics (33). SGLT1 has been identified as the primary pathway for the transport of glucose across the BBM during glucose mass absorption, and SGLT1 is essential for the glucose-induced release of GLP-1 into the peripheral circulation (30,34-37). Downregulation of SGLT1 in the jejunal segment that remains in the alimentary limb after duodenojejunal bypass (DJB) has been found in diabetic rats (38), whereas upregulated expression of SGLT1 (28,39) has been observed in nondiabetic Sprague-Dawley (SD) rats after DJB, with (28) or without (39) upregulated expression of G6Pase and PCK, which are well known as key rate-limiting enzymes of IGNG in the intestine. Therefore, we hypothesized that SGLT1 might play a synergic role in EIA and GBLA that accounts for the mechanisms of glucose remission in MBS. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1769/rc).
Methods
Animals
A protocol was prepared before the study without registration. The study protocol was approved by the Animal Ethics and Welfare Committee of Shenzhen University (No. YSDW202009030), in compliance with Chinese national guidelines for the care and use of animals.
Four 7-week-old male GK rats [Cavens Biogle (Suzhou) Model Animal Research Co. Ltd., Jiangsu, China] and four 7-week-old male Zuker diabetic fatty (ZDF) rats (fa/fa; Charles River Laboratories, Wilmington, MA, USA) were housed individually in a sound-proof environment (to avoid the stressed hyperglycemia) with a specific pathogen-free (SPF) system and acclimatized at a temperature of 20–24 ℃, relative humidity of 50–70%, and 12 hour/12 hour light/dark cycles with a daylight lamp of 40 watts (lights on at 7:00 AM). Standard chow (carbohydrates 58.0%, fat 13.5%, and protein 28.5%) and water were provided ad libitum to rats.
Experimental set-up: tissue harvesting and blood assays
At 10 weeks, the GK rats and ZDF rats weighed 374.4±10.5 and 301.1±6.9 g, respectively, and were ready for specimen harvest. Based on the infusion regime, the GK rats and ZDF rats were assigned to the following groups: the GK-Glu group, ZDF-Glu group, GK-P group, and ZDF-P group (n=2 for each group). The rats in the GK-Glu group and ZDF-Glu group were intragastrically administered glucose solutions as the control groups to the GK-P group and ZDF-P group, in which the rats were infused with a glucose solution mixed with a SGLT1 inhibitor, phlorizin (MedChemExpress, Monmouth Junction, NJ, USA). The research team, with the exception of the principal investigator, was blinded to the assignment of rats to infusion regimes.
The rats were fasted for 18–20 hours before anesthetization. Inhalation anesthesia (2–3% isoflurane in oxygen) was applied. Based on the individual infusion regime, gavage was performed before tissue harvesting. Approximately 30 minutes after gastric infusion, a midline incision was performed. The cecum was displayed to identify the terminal ileum, and the ligament of Treitz was exposed to accurately locate the start of the jejunum.
Segments (2 cm long) from 3 intestinal locations (the horizontal part of duodenum, ~15 cm distal to the Treitz ligament, and ~15 cm proximal to the ileo-cecal) were acquired approximately 90 minutes after infusion. Each segment was divided into 2 1-cm-long loops. The intestinal tissues were harvested for mucosal scrapings and subsequent RNA extraction. The pancreatic tissue was harvested from the caudal junction of the pancreas, and the hepatic tissues were dissected from the middle lobe of the liver 2 hours after gavage was performed. The tissues were immediately snap frozen in liquid nitrogen and stored at ~80 ℃ for subsequent RNA extraction. After the tissue harvesting was completed, the rats were sacrificed and properly buried.
After 18 hours of fasting, blood samples were collected at 9:00–11:00 am to detect glycometabolic parameters fasting plasma glucose (FPG) and fasting insulin (F-ins). Blood glucose (BG) in oral glucose tolerance tests (OGTTs) was assessed by a glucometer (GM9, Analox, Stokesley, UK). Trapezoidal integration was used to calculate the area under the curve (AUC) of OGTT. The AUC was calculated according to the following formula: AUC (mmol·min/L) = BG0h × 0.5 + BG1h × 0.75 + BG2h × 0.25, with BG0h, BG1h, and BG2h indicating BG levels at 0, 1 hour, and 2 hours, respectively. Plasma insulin was measured with insulin enzyme-linked immunosorbent assay (ELISA) kits (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions. The homeostasis model assessment-insulin resistance (HOMA-IR) index was calculated according to the formula (40): HOMA-IR = FPG (mmol/L) × F-ins (pmol/L)/135.
Histological determination
Immunohistochemistry (IHC) was performed to determinate the activity of intestinal SGLT1 expression, while quantitative reverse transcription polymerase chain reaction (RT-qPCR) was applied to detect the following expression levels: SGLT1 mRNA in the duodenum, jejunum ileum, liver, and pancreas; GLP-1R in the jejunum, ileum, and pancreas; and G6Pase and phosphoenolpyruvate carboxykinase-1 (Pck1) in the duodenum, jejunum ileum, and liver.
IHC
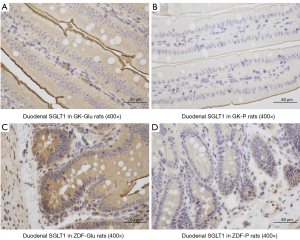
The tissues were harvested and 4-µm thick sections of fixed intestinal tissue were cut and dewaxed. Following rehydration, antigen retrieval was performed in citrate buffer (pH 6.0) and incubated in phosphate-buffered saline (PBS) solution. The sections were blocked by incubation with 3% hydrogen peroxide and incubated in PBS overnight in a refrigerator with affinity-purified rSGLT1-Ab (1:500, Abcam, Cambridge, MA, USA). The sections were rinsed and incubated in PBS for 30 minutes at room temperature, and then dropwise added with secondary antibody horseradish peroxidase (HRP; DAKO, Glostrup, Denmark). Following incubating at 37 ℃ for 60 min, the sections were stained in diaminobenzidine (DAB) for 5–10 min and stained with hematoxylin for 2 min. The stained slides were photographed with a fluorescent Microscope (Eclipse TI-SR, Nikon Corp., Japan). Semi-quantification was performed using the Image J 1.53e software program (Bethesda, MD, USA), via measuring the gray values (Figure 1) that were negatively correlated with the activity of SGLT1 expression, Every single image was analyzed by 2 pathologists who were blinded to the relationships between images and related rats.
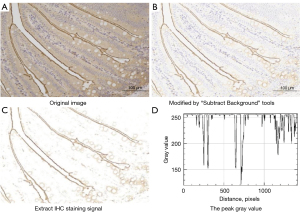
RNA extraction and RT-qPCR
RNA was extracted from frozen tissue samples with an Ambion mirVana mRNA Isolation Kit and the BioPhotometer plus (Eppendorf, Germany) and quantified with a microplate reader (Invitrogen, Carlsbad, CA, USA). Reverse transcription was performed simultaneously on 1.0 µg of RNA from each rat with EasyScript First-Strand cDNA Synthesis SuperMix and oligo-dT (Invitrogen). To facilitate inter- and intragroup comparisons of gene expression, quantitative PCR (qPCR) of all complementary DNA (cDNA) samples were run on a single 96-well plate. The cDNA product was diluted and added to forward and reverse primers [SGTL1, GLP-1R, G6Pase, Pck1, and β-actin (housekeeping gene); Invitrogen; Table 1], together with SYBR Green SuperMix (Applied Biosystems, Waltham, MA, USA). The qPCR was performed in triplicate with diluted cDNA primers and SYBR Green SuperMix using an ABI PRISM® 7500 Sequence Detection System.
Table 1
| Gene | bp | Primer sequence-forward | Primer sequence-reverse |
|---|---|---|---|
| SGTL1 | 170 | CATGCCTAACGGACTTCGA | TGAACAACCTTCCTGCAATC |
| GLP-1R | 150 | ACTCGCGAAGTCCACTCTGA | ACCATAAAGCCCTGGAAGGA |
| G6Pase | 100 | GCAAGAGCTGCAAAGGAGAA | GGCTTCAGCGAGTCAAAGAG |
| Pck1 | 130 | GATCCTGGGCATAACTAACC | ACCCACACATTCAACTTTCC |
| β-actin | 150 | AGGGAAATCGTGCGTGACAT | GAACCGCTCATTGCCGATAG |
RT-qPCR, quantitative reverse transcription polymerase chain reaction; SGTL1, sodium glucose cotransporter 1; GLP-1R, glucagon-like peptide-1 receptor; G6Pase, glucose-6 phosphatase; Pck1, phosphoenolpyruvate carboxykinase-1; β-actin, housekeeping gene.
The thermal cycler conditions used were 5 minutes at 95 ℃, 15 seconds at 95 ℃, and 32 seconds at 60 ℃ for 40 cycles. Dissociation curves were obtained to ensure a single amplicon at 60–95 ℃.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) 21.0 (IBM Corp., Armonk, NJ, USA) and GraphPad Prism 9.0 for Windows (GraphPad Software, San Diego, CA, USA). Data are presented as means ± standard error of mean (SEM) and analyzed using Student’s t-test or 1-way analysis of variance (ANOVA) and post hoc analysis with the least significant difference (LSD) comparison test. Differences were considered statistically significant at P<0.05.
Results
Glucose metabolism
All the animals were tested for comparable weight, FPG, F-ins, and OGTT before harvesting.
No differences in glycometabolic parameters before intragastric infusion (Figure 2)
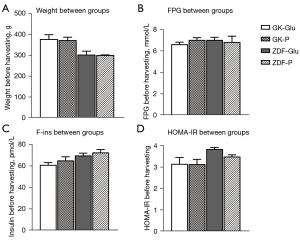
No significant differences in terms of weight, FPG, F-ins, and HOMA-IR were observed between the GK-P group and GK-Glu group (each P>0.05) or between the ZDF-P group and ZDF-Glu group (P>0.05), providing comparability of glucose metabolism between groups after intragastric infusion.
Differences in glucose metabolism between GK rats and ZDF rats were observed (Figure 3)
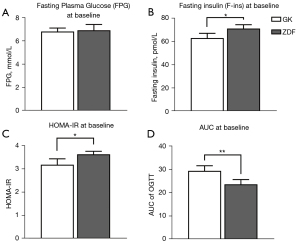
(I) Insulin resistance in ZDF rats was significantly higher than that in GK rats: HOMA-IR in ZDF rats was significantly higher than that in GK rats (P=0.021). (II) The impaired glucose tolerance of GK rats was worse than that of ZDF rats: the AUC area of OGTT in GK rats was larger than that in ZDF rats (P=0.009), whereas the insulin level of GK rats was lower than that of ZDF rats.
Histological analysis
SGLT1 expression
Discrepant expressions of SGLT1 were found in different rat groups
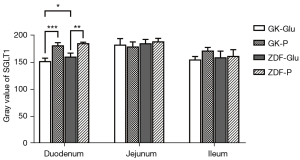
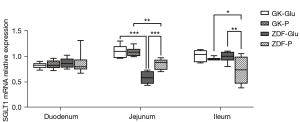
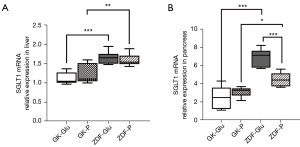
(I) The activity of duodenal SGLT1 in GK rats was higher than that of ZDF rats (P=0.022; Figures 4,5). (II) The expression of SGLT1 mRNA in the jejunum in the GK-Glu group was significantly higher than that in the ZDF-Glu group (1.10±0.05 vs. 0.58±0.05, P=0.000; Figure 6). (III) The expression of SGLT1 mRNA in the pancreas and liver of ZDF rats was significantly higher than that of GK rats: ZDF-Glu group vs. GK-Glu group: 6.91±0.40 vs. 2.50±0.53 (P=0.000) in the pancreas and 1.66±0.07 vs. 1.12±0.06 (P=0.000) in the liver (Figure 7).
The effects of SGLT1 inhibitor
The effects of SGLT1 inhibitor in regulating the expression of SGLT1 in the intestine
(I) The expression of duodenal SGLT1 was effectively inhibited by SGLT1 inhibitors (Figures 4,5) in both GK rats (P=0.000) and ZDF rats (P=0.000). (II) The expression of jejunal SGLT1 mRNA of ZDF rats was effectively inhibited by SGLT1 inhibitors (Figure 6). The jejunal SGLT1 mRNA expression in the ZDF-Glu group was significantly lower than that in the ZDF-P group (P=0.000), but no significant differences were found between the GK-Glu and GK-P groups (P=0.856).
The effects of SGLT1 inhibitor in regulating the expression of SGLT1 mRNA in the liver and pancreas
(I) No significant differences in hepatic SGLT1 mRNA expression were observed between the ZDF-Glu and ZDF-P groups (P=0.550) or between the GK-Glu and GK-P groups (P=0.303; Figure 7A). (II) The SGLT1 inhibitor downregulated the expression of pancreatic SGLT1 mRNA in ZDF rats (ZDF-P group vs. ZDF-Glu group: 4.47±0.32 vs. 6.91±0.40, P=0.000), but there was no regulatory effect on SGLT1 mRNA in GK rats (P=0.244; Figure 7B).
Different effects of SGLT1 inhibitors in inducing the mRNA expression of G6Pase and Pck1 as well as GLP-1R were found
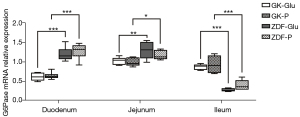
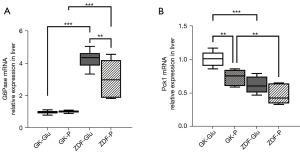
(I) the SGLT1 inhibitor downregulated the expression of GLP-1R mRNA in the jejunum of ZDF rats (the ZDF-P group vs. ZDF-Glu group: 0.55±0.08 vs. 0.95±0.07, P=0.001), but there was no regulatory effect on GLP-1R mRNA in the jejunum of GK rats (P>0.05; Figure 8). (II) No significant differences in intestinal G6Pase mRNA expression were found in the duodenum, jejunum, and ileum between the GK-Glu and GK-P groups (P=0.789, 0.657, and 0.445, respectively) or between the ZDF-Glu and ZDF-P groups (P=0.363, 0.129, and 0.115, respectively; Figure 9). The SGLT1 inhibitor upregulated Pck1 mRNA in the duodenum (ZDF-P group vs. ZDF-Glu group: 1.13±0.09 vs. 0.24±0.03, P=0.000) and the jejunum (ZDF-P group vs. ZDF-Glu group: 1.47±0.07 vs. 1.18±0.11, P=0.038) of ZDF rats, but no significant differences in intestinal Pck1 mRNA were found between the GK-P group and GK-Glu group (Figure 10). (III) The SGLT1 inhibitor downregulated G6Pase mRNA in the liver of ZDF rats (ZDF-P group vs. ZDF-Glu group: 3.05±0.53 vs. 4.27±0.23, P=0.005; Figure 11A) and Pck1 mRNA expression in GK rats (GK-P group vs. GK-Glu group: 0.74±0.05 vs. 1.00±0.05, P=0.001; Figure 11B). (IV) The SGLT1 inhibitor upregulated GLP-1R mRNA in the pancreas of ZDF rats (Figure 8). Pancreatic GLP-1R mRNA expression in the ZDF-P group was significantly higher than that in the ZDF-Glu group (1.37±0.24 vs. 0.80±0.07, P=0.021), but no significant differences were found between the GK-Glu and GK-P groups (P=0.097).


Discussion
The incidence of diabetes is increasing and finding effective treatment has become an urgent and important issue. MBS, represented by RYGB, has achieved encouraging results in the treatment of diabetes, with a postoperative remission rate as high as 83.7–98.9% (41). MBS is being performed in nonobese diabetes more and more (41,42). In 2008, the American Society for Bariatric Surgery (ASBS) was renamed as the American Society for Metabolic & Bariatric Surgery (ASMBS), reflecting the widespread acceptance of the surgical treatment for diabetes by specialists (43). However, the mechanism of MBS in the treatment of diabetes is not fully understood, and the indications of diabetic patients for MBS (i.e., the definition of “surgical diabetes”) are not well defined (44).
The “SGLT1 bridge” hypothesis originally proposed and our interpretation of inconsistencies
The therapeutic mechanism of surgery cannot be convincingly explained by the classic theory of EIA based on GLP-1 and GBLA initiated by IGNG. Postoperative glucose improvement is observed in RYGB with reserved or disconnected vagus nerve (44), raising questions about the ability of the GBLA hypothesis to explain the mechanism of the surgical treatment effect. As for the EIA hypothesis, numerous studies (4-11,13-15) have raised doubts on the importance of GLP-1 to diabetic remission in MBS, which is consistent with the findings of our previous research (15), which suggested that increased GLP-1 may be an “intermediate step” in glucose improvement after MBS. As for identifying an “upstream factor”, the molecular mechanisms of small intestinal nutrient sensing in metabolic homeostasis have physiological and pathological impact as well as therapeutic potential in diabetes (45). Our team believes that it is likely to be SGLT1 that induces the improvement of glycometabolism synergically via both the EIA and GBLA pathways (44). For the sake of description, we summarize it as the “SGLT1 bridge” hypothesis, which implies that SGLT1 acts as a bridge-like mechanistic link between EIA and GBLA and plays a synergistic role in these 2 axes.
Controversially, in some studies of rats after MBS, i.e., DJB surgery, the expression of SGTL1 in the alimentary limb showed either upregulation (28,39) (in nondiabetic rats) or downregulation (in diabetic rats) (38). Consideration that SGLT1 expression may be related to the pathophysiological conditions of the diabetic rats has not received enough attention in previous metabolic studies. We hypothesized that the expression of SGLT1 is discrepant in different diabetic profiles. Consistent with our hypothesis, Chichger et al. (46) found that compared with Wistar rats with normal BG, enhanced expression of multiple sodium-glucose transporters (including SGLT1) was evident in the renal proximal tubule of diabetic GK rats, whereas only the expression of SGLT1 was enhanced in the proximal tubule of streptozotocin-induced diabetic rats. However, Chichger’s study only examined SGLT1 expression in the kidney. Herein, we investigated the expression of SGLT1 in different diabetic rats in multiple digestive system organs, including the intestine, the liver, and the pancreas.
Diabetic rats in distinct pathophysiological conditions present discrepant glycometabolic profiles
Several rat models have been applied to research on metabolic mechanisms and SGLT1 (28,46-55). ZDF rats are widely used to study physiology and pathology in obese diabetes (56-64), representing early-stage type 2 diabetes well. GK rats, a spontaneously nonobese diabetic rat model that exhibits stable hyperglycemia, marked glucose intolerance, insulin resistance, and impaired glucose-induced insulin secretion (65), are considered an ideal model for the advanced stage of diabetes (46,66,67). Therefore, we chose ZDF rats and GK rats to investigate the differences that might result from the distinct pathophysiological conditions of diabetes.
The different glycometabolic profiles in rats representing different stages of diabetes were verified as follows: (I) the insulin resistance of ZDF rats was significantly higher than that of GK rats (Figure 3C), which may have been attributed to the obesity of ZDF rats that the nonobese GK rats did not possess; and (II) the impaired glucose tolerance in OGTT was more severe in GK rats than ZDF rats (Figure 3D), and the fasting insulin level was lower in GK rats than in ZDF rats (Figure 3B), consistent with progression in type 2 diabetes. In humans with a long duration of type 2 diabetes, further impaired glucose tolerance and a deficiency of insulin secretion may occur. Therefore, the ZDF rats and GK rats selected for the present study simulated the models of early and advanced stages of diabetes well, which contributed to the credibility of the conclusion.
Distinct pathophysiological conditions might account for the discrepant expression of SGLT1 in diabetic rats
The notion that MBS has no effect on BG (41,42,44) is still hard to explain in clinical practice, and the indications of diabetic patients for surgery treatment have not yet been well defined. A high body mass index (BMI) is the most frequently considered indication, and the duration of diabetes is another important index that has been proposed as a significant predictor of prognosis (42), with the longer the duration of diabetes, the worse the efficacy of MBS. Nevertheless, there is no clear and reasonable explanation for the poor efficacy of surgery in patients with long duration of diabetes. It is generally believed that advanced type 2 diabetes is prone to secretion deficiency of insulin that is similar to type 1 diabetes. The “ABCD score system” (68,69) [comprising age (A), BMI (B), C peptide (C), and duration (D)] is applied to diabetic patients who are candidates for surgery and has improved the overall efficacy of surgery in the treatment of diabetes. However, invalid improvement and remission after MBS continue to occur (41). We hypothesized that the expression of SGLT1 might be different in diabetic rats with distinct pathophysiology, which might account for the remission of diabetes postoperatively.
SGLT1 is expressed mainly in the small intestine, with peak activity in the duodenum and less expression in the distal small intestine (70), which was inconsistent with the diabetic rats in the present study, indicating that the expression of SGLT1 in diabetic rats may possess inherent pathophysiological conditions. Higher expression of SGLT1 in diabetes has been reported (30,71-76). We compared rats in 2 distinct pathophysiological conditions, representing early and advanced stages of diabetes, and found that the activity of SGLT1 expressed in the duodenum of GK rats was higher than that in ZDF rats after glucose gavage (Figure 4). Further, the mRNA expression of SGLT1 in the jejunum of GK rats was higher than in ZDF rats (Figure 6), indicating the probability that the longer the duration of diabetes, the higher the expression of SGLT1 in the “foregut”. Differences in SGLT1 expression levels should be regarded as distinct pathophysiological states of diabetes (75-77) and is worthy of further study involving humans with different duration of diabetes.
SGLT1 may synergically regulate GLP-1 expression and gluconeogenesis (GNG)
The expression of SGLT1 has an obvious circadian rhythm (52,71); its peak expression at 9–10 am is 2–3 times higher (protein level) or 5 times higher (mRNA) than the lowest point, and its half-life is about 2 hours (58). Based on these inherent properties of SGLT1, the tissues of the small intestine were harvested at 10:30 am, and the tissues of the liver and pancreas were harvested at 11:00 am. It has been reported (52,78) that intestinal SGLT1 expression is independent of local luminal factors, not affected by paracrine of the pancreas, and seems to be regulated only by circadian rhythms. Taken together, it is reasonable to speculate that, as the “first gateway” for food contact in the intestine, SGLT1 could be considered “upstream” of IGNG and intestinal hormones. Phlorizin, a classic SGLT1 inhibitor we chose to verify the regulatory effect of SGLT1 in vivo, can effectively inhibit the expression of SGLT1 in the intestine.
SGLT1 plays an important role in mediating glucose-dependent GLP-1 expression (34,35,79), both in the intestine and pancreas (15). Gorboulev et al. (30) found reduced GLP-1 expression in SGLT1-gene knockout mice compared with normal mice, indicating that SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent GLP-1 secretion. GSK-1614235, a highly selective SGLT1 inhibitor, can reduce glucose-dependent GLP-1 level in volunteers with normal BG (80). Multiple regulatory effects of SGLT1 inhibitor on GLP-1R were shown in our study (Figure 8): (I) the decreased expression of GLP-1R in the jejunum of ZDF rats after the use of SGLT1 inhibitor; and (II) upregulated GLP-1R expression in the pancreas of ZDF rats. SGLT1 regulation of GLP-1R expression in the intestine and the pancreas of ZDF rats was confirmed, whereas the regulatory effect of SGLT1 on the expression of intestinal and pancreatic GLP-1R mRNA was not shown in GK rats (Figure 8). Such differences might have resulted from the failure of an inhibiting effect by phlorizin in the jejunum and the ileum of GK rats (Figure 6), while the expression of SGLT1 mRNA in the jejunum and ileum of ZDF rats was effectively regulated by the SGLT1 inhibitor (Figure 6). Combined with the finding mentioned above that the activity of SGLT1 expression in the duodenum of GK rats was stronger than that in ZDF rats (Figures 4,5), we speculated that unlike ZDF rats in early diabetes, the regulation pathway of SGLT1- GLP-1R in GK rats with advanced diabetes may have been blocked. The differences seen in diabetic rats with varying durations may have been due to complex pathophysiological conditions, which may account for the lower remission rate or higher recurrence rate after surgery in diabetic patients with longer durations (42,77). Therefore, an intact SGLT1-GLP-1R regulation pathway might be an important indication for the “surgical diabetes” (44) that we have proposed.
G6Pase and Pck1 are the key rate-limiting enzymes of GNG, and expression will be enhanced when hungry (81), which plays a crucial role in EGP and the maintenance of BG homeostasis. Troy et al. (26) showed that IGNG was a key factor for early metabolic changes after gastric bypass in mice, which is consistent with other studies involving rats (10,28,29,39). This means that the GBLA pathway originating from IGNG and terminating at HGNG has been preliminarily validated in animal models of metabolic bariatric surgery.
However, inconsistencies remain regarding whether SGLT1 is located “upstream” of IGNG. After DJB surgery, the alimentary limb of rats with normal BG presented high expressions of SGLT1 with upregulation of IGNG (28,39) and downregulation of HGNG (28), whereas downregulated expression of intestinal SGLT1 and approximately a half-reduced function of glucose absorption were found in streptozotocin-induced diabetic rats (38). We believe that the inconsistencies may be caused by the glucose metabolism status of the rat models. Therefore, the ideal rat models representing early and advanced diabetes were adopted in the present study to explore the effect of SGLT1 on GBLA. The SGLT1 inhibitors downregulated activity of duodenal SGLT1 in both ZDF rats and GK rats (Figures 4,5), which resulted in upregulated expressions of Pck1 mRNA in the duodenum and jejunum of ZDF rats (Figure 10), whereas no regulatory effect of SGLT1 inhibitor on key enzymes of IGNG was found in GK rats (Figures 9,10). Additionally, the expression of G6Pase (Figure 11A) and Pck1 mRNA (Figure 11B) in the liver of ZDF rats was downregulated without any effect on the expression of SGLT1 mRNA in the liver of ZDF rats (Figure 7A), suggesting that the effect of SGLT1 inhibitor on HGNG of ZDF rats was a “remote” induction rather than a local regulation, likely resulting from the IGNG-GBLA-HGNG pathway. These results indicated that SGLT1 might have had a negative regulatory effect on IGNG, and a positive regulatory effect on HGNG in ZDF rats. However, such regulatory effects on GNG were not found in GK rats, and combined with the aforementioned finding that GK rats had more severely impaired glucose tolerance than ZDF rats (Figure 3D), the results suggested that the IGNG-GBLA-HGNG pathway in GK rats with advanced diabetes may have been blunted or even blocked. Therefore, we speculated that the unsatisfactory improvement of glucose metabolism or easy relapse of diabetes after surgery may be caused by the failure of induction on the GBLA pathway due to certain pathophysiological changes in advanced diabetes. A well-retained SGLT1-IGNG-GBLA-HGNG pathway might be important and should be taken as one of the indications for “surgical diabetes”.
Taken together, our results suggested that SGLT1 may synergistically induce both the EIA and GBLA pathways to improve glucose metabolism in a type of diabetes referred to as “surgical diabetes” and preliminarily validated the “SGLT1 bridge” hypothesis we have proposed. However, the exact mechanism of the “SGLT1 bridge” as an indication for “surgical diabetes” remains to be elaborated. The role of SGLT1 in “surgical diabetes” is worthy of further study.
Acknowledgments
Funding: The study was supported by the Natural Science Foundation of Shenzhen University General Hospital (No. SUGH2019QD016), and supported by Sanming Project of Medicine in Shenzhen (No. SZSM202111002).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1769/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1769/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1769/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Animal Ethics and Welfare Committee of Shenzhen University (No. YSDW202009030), in compliance with Chinese national guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chang SH, Stoll CR, Song J, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg 2014;149:275-87. [Crossref] [PubMed]
- Oh TJ, Lee HJ, Cho YM. Ileal Transposition Decreases Plasma Lipopolysaccharide Levels in Association with Increased L Cell Secretion in Non-obese Non-diabetic Rats. Obes Surg 2016;26:1287-95. [Crossref] [PubMed]
- Chamberlain JJ, Rhinehart AS, Shaefer CF Jr, et al. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med 2016;164:542-52. [Crossref] [PubMed]
- Seeley RJ, Chambers AP, Sandoval DA. The role of gut adaptation in the potent effects of multiple bariatric surgeries on obesity and diabetes. Cell Metab 2015;21:369-78. [Crossref] [PubMed]
- Jiménez A, Casamitjana R, Viaplana-Masclans J, et al. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care 2013;36:2062-9. [Crossref] [PubMed]
- Jiménez A, Mari A, Casamitjana R, et al. GLP-1 and glucose tolerance after sleeve gastrectomy in morbidly obese subjects with type 2 diabetes. Diabetes 2014;63:3372-7. [Crossref] [PubMed]
- Salehi M, Gastaldelli A, D'Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 2014;146:669-680.e2. [Crossref] [PubMed]
- Jiménez A, Casamitjana R, Flores L, et al. GLP-1 and the long-term outcome of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery in morbidly obese subjects. Ann Surg 2013;257:894-9. [Crossref] [PubMed]
- Mokadem M, Zechner JF, Margolskee RF, et al. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab 2014;3:191-201. [Crossref] [PubMed]
- Mithieux G. A synergy between incretin effect and intestinal gluconeogenesis accounting for the rapid metabolic benefits of gastric bypass surgery. Curr Diab Rep 2012;12:167-71. [Crossref] [PubMed]
- Patel RT, Shukla AP, Ahn SM, et al. Surgical control of obesity and diabetes: the role of intestinal vs. gastric mechanisms in the regulation of body weight and glucose homeostasis. Obesity (Silver Spring) 2014;22:159-69. [Crossref] [PubMed]
- Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol 2013;10:575-84. [Crossref] [PubMed]
- Reinehr T, Roth CL, Schernthaner GH, et al. Peptide YY and glucagon-like peptide-1 in morbidly obese patients before and after surgically induced weight loss. Obes Surg 2007;17:1571-7. [Crossref] [PubMed]
- Wilson-Pérez HE, Chambers AP, Ryan KK, et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes 2013;62:2380-5. [Crossref] [PubMed]
- Zhu H, Wang H, Zheng Z, et al. Ileal transposition rapidly improves glucose tolerance and gradually improves insulin resistance in non-obese type 2 diabetic rats. Gastroenterol Rep (Oxf) 2018;6:291-7. [Crossref] [PubMed]
- Morrow NM, Hanson AA, Mulvihill EE. Distinct Identity of GLP-1R, GLP-2R, and GIPR Expressing Cells and Signaling Circuits Within the Gastrointestinal Tract. Front Cell Dev Biol 2021;9:703966. [Crossref] [PubMed]
- Kohli R, Kirby M, Setchell KD, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol 2010;299:G652-60. [Crossref] [PubMed]
- Oh TJ, Ahn CH, Cho YM. Contribution of the distal small intestine to metabolic improvement after bariatric/metabolic surgery: Lessons from ileal transposition surgery. J Diabetes Investig 2016;7:94-101. [Crossref] [PubMed]
- Mithieux G. Influence of diabetes surgery on a gut-brain-liver axis regulating food intake and internal glucose production. Nutr Hosp 2013;28:109-14. [PubMed]
- Mithieux G, Misery P, Magnan C, et al. Portal sensing of intestinal gluconeogenesis is a mechanistic link in the diminution of food intake induced by diet protein. Cell Metab 2005;2:321-9. [Crossref] [PubMed]
- Mingrone G, Castagneto-Gissey L. Mechanisms of early improvement/resolution of type 2 diabetes after bariatric surgery. Diabetes Metab 2009;35:518-23. [Crossref] [PubMed]
- Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006;244:741-9. [Crossref] [PubMed]
- Rajas F, Bruni N, Montano S, et al. The glucose-6 phosphatase gene is expressed in human and rat small intestine: regulation of expression in fasted and diabetic rats. Gastroenterology 1999;117:132-9. [Crossref] [PubMed]
- Mithieux G, Bady I, Gautier A, et al. Induction of control genes in intestinal gluconeogenesis is sequential during fasting and maximal in diabetes. Am J Physiol Endocrinol Metab 2004;286:E370-5. [Crossref] [PubMed]
- Mithieux G, Rajas F, Gautier-Stein A. A novel role for glucose 6-phosphatase in the small intestine in the control of glucose homeostasis. J Biol Chem 2004;279:44231-4. [Crossref] [PubMed]
- Troy S, Soty M, Ribeiro L, et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab 2008;8:201-11. [Crossref] [PubMed]
- Goncalves D, Barataud A, De Vadder F, et al. Bile Routing Modification Reproduces Key Features of Gastric Bypass in Rat. Ann Surg 2015;262:1006-15. [Crossref] [PubMed]
- Kim M, Son YG, Kang YN, et al. Changes in glucose transporters, gluconeogenesis, and circadian clock after duodenal-jejunal bypass surgery. Obes Surg 2015;25:635-41. [Crossref] [PubMed]
- Sun D, Wang K, Yan Z, et al. Duodenal-jejunal bypass surgery up-regulates the expression of the hepatic insulin signaling proteins and the key regulatory enzymes of intestinal gluconeogenesis in diabetic Goto-Kakizaki rats. Obes Surg 2013;23:1734-42. [Crossref] [PubMed]
- Gorboulev V, Schürmann A, Vallon V, et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012;61:187-96. [Crossref] [PubMed]
- Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91:733-94. [Crossref] [PubMed]
- Stearns AT, Balakrishnan A, Tavakkolizadeh A. Impact of Roux-en-Y gastric bypass surgery on rat intestinal glucose transport. Am J Physiol Gastrointest Liver Physiol 2009;297:G950-7. [Crossref] [PubMed]
- Dyer J, Wood IS, Palejwala A, et al. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol 2002;282:G241-8. [Crossref] [PubMed]
- Reimann F, Habib AM, Tolhurst G, et al. Glucose sensing in L cells: a primary cell study. Cell Metab 2008;8:532-9. [Crossref] [PubMed]
- Gribble FM, Williams L, Simpson AK, et al. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 2003;52:1147-54. [Crossref] [PubMed]
- Nielsen LB, Ploug KB, Swift P, et al. Co-localisation of the Kir6.2/SUR1 channel complex with glucagon-like peptide-1 and glucose-dependent insulinotrophic polypeptide expression in human ileal cells and implications for glycaemic control in new onset type 1 diabetes. Eur J Endocrinol 2007;156:663-71. [Crossref] [PubMed]
- Fujita Y, Wideman RD, Speck M, et al. Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. Am J Physiol Endocrinol Metab 2009;296:E473-9. [Crossref] [PubMed]
- Jurowich CF, Rikkala PR, Thalheimer A, et al. Duodenal-jejunal bypass improves glycemia and decreases SGLT1-mediated glucose absorption in rats with streptozotocin-induced type 2 diabetes. Ann Surg 2013;258:89-97. [Crossref] [PubMed]
- Takayama H, Ohta M, Tada K, et al. Additional effects of duodenojejunal bypass on glucose metabolism in a rat model of sleeve gastrectomy. Surg Today 2019;49:637-44. [Crossref] [PubMed]
- Ruijgrok C, Dekker JM, Beulens JW, et al. Size and shape of the associations of glucose, HbA1c, insulin and HOMA-IR with incident type 2 diabetes: the Hoorn Study. Diabetologia 2018;61:93-100. [Crossref] [PubMed]
- Ruan X, Zhang W, Cai H, et al. Sleeve gastrectomy with duodenojejunal end-to-side anastomosis in the treatment of type 2 diabetes: the initial experiences in a Chinese population with a more than 4-year follow-up. Surg Obes Relat Dis 2017;13:1683-91. [Crossref] [PubMed]
- Jiang F, Zhu H, Zheng X, et al. Duodenal-jejunal bypass for the treatment of type 2 diabetes in Chinese patients with an average body mass index<24 kg/m2. Surg Obes Relat Dis 2014;10:641-6. [Crossref] [PubMed]
- Rubino F, Shukla A, Pomp A, et al. Bariatric, metabolic, and diabetes surgery: what's in a name? Ann Surg 2014;259:117-22. [Crossref] [PubMed]
- Zhu H, Jiang F. Gastrointestinal sodium-glucose cotransporter 1 may mediate metabolic bariatric surgery to improve blood glucose. Chin J Obes Metab Dis 2016;2:80-4.
- Duca FA, Waise TMZ, Peppler WT, et al. The metabolic impact of small intestinal nutrient sensing. Nat Commun 2021;12:903. [Crossref] [PubMed]
- Chichger H, Cleasby ME, Srai SK, et al. Experimental type II diabetes and related models of impaired glucose metabolism differentially regulate glucose transporters at the proximal tubule brush border membrane. Exp Physiol 2016;101:731-42. [Crossref] [PubMed]
- Cavin JB, Couvelard A, Lebtahi R, et al. Differences in Alimentary Glucose Absorption and Intestinal Disposal of Blood Glucose After Roux-en-Y Gastric Bypass vs Sleeve Gastrectomy. Gastroenterology 2016;150:454-64.e9. [Crossref] [PubMed]
- Jurowich CF, Otto C, Rikkala PR, et al. Ileal Interposition in Rats with Experimental Type 2 Like Diabetes Improves Glycemic Control Independently of Glucose Absorption. J Diabetes Res 2015;2015:490365. [Crossref] [PubMed]
- Xu G, Du F, Kuo GH, et al. 5,5-Difluoro- and 5-Fluoro-5-methyl-hexose-based C-Glucosides as potent and orally bioavailable SGLT1 and SGLT2 dual inhibitors. Bioorg Med Chem Lett 2020;30:127387. [Crossref] [PubMed]
- Kuhre RE, Ghiasi SM, Adriaenssens AE, et al. No direct effect of SGLT2 activity on glucagon secretion. Diabetologia 2019;62:1011-23. [Crossref] [PubMed]
- Chae H, Augustin R, Gatineau E, et al. SGLT2 is not expressed in pancreatic α- and β-cells, and its inhibition does not directly affect glucagon and insulin secretion in rodents and humans. Mol Metab 2020;42:101071. [Crossref] [PubMed]
- Stearns AT, Balakrishnan A, Rhoads DB, et al. Diurnal expression of the rat intestinal sodium-glucose cotransporter 1 (SGLT1) is independent of local luminal factors. Surgery 2009;145:294-302. [Crossref] [PubMed]
- Sharma R, Kaur J, Chauhan SS, et al. Gestational diabetes affects postnatal development of transport and enzyme functions in rat intestine. Mol Cell Biochem 2012;361:71-7. [Crossref] [PubMed]
- Debnam ES, Smith MW, Sharp PA, et al. The effects of streptozotocin diabetes on sodium-glucose transporter (SGLT1) expression and function in rat jejunal and ileal villus-attached enterocytes. Pflugers Arch 1995;430:151-9. [Crossref] [PubMed]
- Sabino-Silva R, Alves-Wagner AB, Burgi K, et al. SGLT1 protein expression in plasma membrane of acinar cells correlates with the sympathetic outflow to salivary glands in diabetic and hypertensive rats. Am J Physiol Endocrinol Metab 2010;299:E1028-37. [Crossref] [PubMed]
- Bhutta HY, Deelman TE, le Roux CW, et al. Intestinal sweet-sensing pathways and metabolic changes after Roux-en-Y gastric bypass surgery. Am J Physiol Gastrointest Liver Physiol 2014;307:G588-93. [Crossref] [PubMed]
- Oguma T, Kuriyama C, Nakayama K, et al. The effect of combined treatment with canagliflozin and teneligliptin on glucose intolerance in Zucker diabetic fatty rats. J Pharmacol Sci 2015;127:456-61. [Crossref] [PubMed]
- Du F, Hinke SA, Cavanaugh C, et al. Potent Sodium/Glucose Cotransporter SGLT1/2 Dual Inhibition Improves Glycemic Control Without Marked Gastrointestinal Adaptation or Colonic Microbiota Changes in Rodents. J Pharmacol Exp Ther 2018;365:676-87. [Crossref] [PubMed]
- Palaniappan B, Arthur S, Sundaram VL, et al. Inhibition of intestinal villus cell Na/K-ATPase mediates altered glucose and NaCl absorption in obesity-associated diabetes and hypertension. FASEB J 2019;33:9323-33. [Crossref] [PubMed]
- Vangoitsenhoven R, Mulya A, Mosinski JD, et al. Effects of gastric bypass surgery on expression of glucose transporters and fibrotic biomarkers in kidney of diabetic fatty rats. Surg Obes Relat Dis 2020;16:1242-8. [Crossref] [PubMed]
- Wachal Z, Szilágyi A, Takács B, et al. Improved Survival and Retinal Function of Aging ZDF Rats in Long-Term, Uncontrolled Diabetes by BGP-15 Treatment. Front Pharmacol 2021;12:650207. [Crossref] [PubMed]
- Oguma T, Nakayama K, Kuriyama C, et al. Intestinal Sodium Glucose Cotransporter 1 Inhibition Enhances Glucagon-Like Peptide-1 Secretion in Normal and Diabetic Rodents. J Pharmacol Exp Ther 2015;354:279-89. [Crossref] [PubMed]
- Bhutta HY, Deelman TE, Ashley SW, et al. Disrupted circadian rhythmicity of the intestinal glucose transporter SGLT1 in Zucker diabetic fatty rats. Dig Dis Sci 2013;58:1537-45. [Crossref] [PubMed]
- Shibazaki T, Tomae M, Ishikawa-Takemura Y, et al. KGA-2727, a novel selective inhibitor of a high-affinity sodium glucose cotransporter (SGLT1), exhibits antidiabetic efficacy in rodent models. J Pharmacol Exp Ther 2012;342:288-96. [Crossref] [PubMed]
- Yan Z, Chen W, Liu S, et al. Myocardial insulin signaling and glucose transport are up-regulated in Goto-Kakizaki type 2 diabetic rats after ileal transposition. Obes Surg 2012;22:493-501. [Crossref] [PubMed]
- Jurysta C, Nicaise C, Giroix MH, et al. Comparison of GLUT1, GLUT2, GLUT4 and SGLT1 mRNA expression in the salivary glands and six other organs of control, streptozotocin-induced and Goto-Kakizaki diabetic rats. Cell Physiol Biochem 2013;31:37-43. [Crossref] [PubMed]
- Satoh T, Igarashi M, Yamada S, et al. Inhibitory effect of black tea and its combination with acarbose on small intestinal α-glucosidase activity. J Ethnopharmacol 2015;161:147-55. [Crossref] [PubMed]
- Naitoh T, Kasama K, Seki Y, et al. Efficacy of Sleeve Gastrectomy with Duodenal-Jejunal Bypass for the Treatment of Obese Severe Diabetes Patients in Japan: a Retrospective Multicenter Study. Obes Surg 2018;28:497-505. [Crossref] [PubMed]
- Lee WJ, Almulaifi A, Tsou JJ, et al. Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus: predicting the success by ABCD score. Surg Obes Relat Dis 2015;11:991-6. [Crossref] [PubMed]
- Yoshikawa T, Inoue R, Matsumoto M, et al. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem Cell Biol 2011;135:183-94. [Crossref] [PubMed]
- Balakrishnan A, Stearns AT, Rounds J, et al. Diurnal rhythmicity in glucose uptake is mediated by temporal periodicity in the expression of the sodium-glucose cotransporter (SGLT1). Surgery 2008;143:813-8. [Crossref] [PubMed]
- Lehmann A, Hornby PJ. Intestinal SGLT1 in metabolic health and disease. Am J Physiol Gastrointest Liver Physiol 2016;310:G887-98. [Crossref] [PubMed]
- Koepsell H. Glucose transporters in the small intestine in health and disease. Pflugers Arch 2020;472:1207-48. [Crossref] [PubMed]
- Röder PV, Geillinger KE, Zietek TS, et al. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS One 2014;9:e89977. [Crossref] [PubMed]
- Sundaram S, Borthakur A. Altered intestinal epithelial nutrient transport: an underappreciated factor in obesity modulated by diet and microbiota. Biochem J 2021;478:975-95. [Crossref] [PubMed]
- Gromova LV, Polozov AS, Savochkina EV, et al. Effect of Type 2 Diabetes and Impaired Glucose Tolerance on Digestive Enzymes and Glucose Absorption in the Small Intestine of Young Rats. Nutrients 2022;14:385. [Crossref] [PubMed]
- Jiang B, Wang H, Li N, et al. Role of Proximal Intestinal Glucose Sensing and Metabolism in the Blood Glucose Control in Type 2 Diabetic Rats After Duodenal Jejunal Bypass Surgery. Obes Surg 2022;32:1119-29. [Crossref] [PubMed]
- Gruzdkov AA, Dmitrieva YV, Alekseeva AS, et al. Evaluation of glucose absorption level in the small intestine of different rat strains under natural conditions. J Evol Biochem Physiol 2018;54:308-15. [Crossref]
- Parker HE, Adriaenssens A, Rogers G, et al. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia 2012;55:2445-55. [Crossref] [PubMed]
- Dobbins RL, Greenway FL, Chen L, et al. Selective sodium-dependent glucose transporter 1 inhibitors block glucose absorption and impair glucose-dependent insulinotropic peptide release. Am J Physiol Gastrointest Liver Physiol 2015;308:G946-54. [Crossref] [PubMed]
- Sala PC, Torrinhas RS, Heymsfield SB, et al. Type 2 diabetes mellitus: a possible surgically reversible intestinal dysfunction. Obes Surg 2012;22:167-76. [Crossref] [PubMed]
(English Language Editor: A. Muijlwijk)


