
A case report and literature review: diagnosis and treatment of human immunodeficiency virus coinfected with visceral leishmania by metagenomic next-generation sequencing in China
Introduction
Acquired immunodeficiency syndrome (AIDS) is a serious infectious disease caused by the human immunodeficiency virus (HIV) that primarily attacks the immune system (especially CD4+ T lymphocytes, monocyte macrophages, and dendritic cells) (1). The destruction of the immune system, especially cellular immunity, significantly increases the probability of AIDS patients developing opportunistic infections such as viruses, bacteria, fungi, and parasites. Severe and multiple infections are the main clinical symptoms of AIDS patients and the leading cause of death, causing a great economic and psychological burden to the patient’s family and society.
Leishmaniasis is a vector-borne disease caused by protozoan parasites of the genus Leishmania. It is widely distributed in 88 tropical, subtropical, and temperate countries, with more than 350 million people at risk. Visceral leishmaniasis (VL) has become a significant opportunistic infection in HIV type 1 (HIV-1)-infected patients in the epidemic regions (2). The first case of Leishmania/HIV coinfection was reported in 1985 (3), and subsequent cases of coinfection have been reported in several countries. A previous study have shown that AIDS increases the risk of VL by 100 to 1,000 times in leishmaniasis-affected areas (4).
All suspected leishmania infection can be diagnosed in three ways: clinical, immunological and pathogenic; However, there is no specificity in clinical diagnosis after infection and it is difficult to make a diagnosis only by clinical symptoms. At present, routine diagnosis is still mainly based on experimental diagnosis: rapid immunoassay based on rK39 antigen. The detection of Leishmania is the gold standard for the diagnosis of leishmaniasis. Microscopic examination of bone marrow, lymph node, and spleen aspirates is the most reliable method for confirming the diagnosis of VL. Currently, the commonly used efficacious agents for leishmaniasis include antimony and amphotericin B (AMB).
Failure to take rK39 test into account by clinicians, inability to perform rK39 test in the area, or inability of clinicians to identify the pathogen under microscopy may result in misdiagnosis or missed diagnosis. Therefore, Leishmania infection, especially in the case of coinfection with HIV, should warrant a much higher degree of research attention.
In this case, we finally confirmed leishmania infection with metagenomic next-generation sequencing (mNGS), and the patient had a good therapeutic effect against leishmania infection. In this paper, we reviewed the transmission, pathogenesis, clinical manifestations, epidemiology, diagnosis and treatment of Leishmaniasis, and introduced mNGS technology and its advantages in diagnosis. We recommend the use of mNGS for etiological screening in patients who have multiple negative etiological tests and not responded to multiple empirical anti-infective therapies.It is expected that readers can have a deeper understanding of Leishmaniasis and adopt mNGS technology for early diagnosis and treatment at the appropriate time, so as to achieve the benefit of patients. We present the following article in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1351/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. We report the case of a 44-year-old male farmer born in Yunnan Province, who had been working as a cotton picker in the Xinjiang Autonomous Region for many years. His main symptom was recurrent fever for more than 1 month. The patient visited local clinics and hospitals, completed relevant tests, and received symptomatic treatment (the specific test results and treatment plans were not available), but the fever remained uncontrolled.
The patient then presented to the West China Hospital of Sichuan University and completed blood tests, computed tomography (CT), and other examinations. The patient’s test results are shown in Table 1.
Table 1
| Parameters | Reports | Comments |
|---|---|---|
| Hb | 60 g/L | Decreased |
| RBC count | 2.26×109/L | Decreased |
| MCV | 99.6 fL | Normal |
| TLC | 1.59×109/L | Decreased |
| Neutrophils | 70.4% | Normal |
| Platelets | 21×109/L | Decreased |
| Total bilirubin | 12.3 μmol/L | Normal |
| Direct bilirubin | 6.7 mol/L | Normal |
| GGT | 28 IU/L | Normal |
| ALT | 12 IU/L | Normal |
| ALP | 159 IU/L | Normal |
| AST | 23 IU/L | Normal |
| LDH | 172 IU/L | Normal |
| Ferritin | 762 ng/mL | Raised |
| Triglyceride | 0.7 mmol/L | Normal |
| CD3 COUNT | 338 cell/μL | Decreased |
| CD4 COUNT | 12 cell/μL | Decreased |
| CD8 COUNT | 316 cell/μL | Normal |
| FIB | 1.06 g/L | Normal |
| IL-2R | 870 μ/mL | Raised |
| HIV-RNA | 1.29×105 copies/mL | Raised |
| EB-DNA | 1.64×102 copies/mL | Raised |
| Galactomannan | 0.52 GMI | Raised |
| 1-3-β-D polyglucosan | 44.2 pg/mL | Normal |
| TB-IGRA | 1.64 pg/mL | Normal |
Hb, hemoglobin; RBC, red blood cell; MCV, mean corpuscular volume; TLC, total leukocyte count; GGT, gamma-glutamyl transferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; FIB, fibrinogen; IL-2R, interleukin-2 receptor; HIV-RNA, human immunodeficiency virus RNA; EB-DNA, Epstein-Barr virus DNA; TB-IGRA, interferon-gamma release assay for mycobacterium tuberculosis.
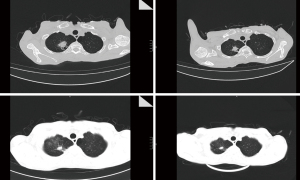
Of the blood tests, (1,3)-β-D glucan assay, cryptococcal antigen, TORCH (toxoplasma, rubella virus, cytomegalovirus, herpes simplex virus), TB-IGRA (tuberculosis interferon-gamma release assay), and multiple blood cultures were negative. The main positive findings were whole blood cytopenia, reduced CD4+ T lymphocyte count, and positive HIV and EBV replication (Table 1). The CT showed a soft tissue shadow in the apical segment of the upper lobe of the right lung, with an area of about 2.5 cm × 2.0 cm, surrounded by patchy shadow and ground glass shadow with a blurred border. The other nodular shadow was scattered in both lungs, partly with cavities, and the largest one was located in the postapical segment of the upper lobe of the left lung, with a length of about 1.0 cm. The large nodules were located in the posterior part of the left upper lobe with a diameter of about 1.0 cm (Figure 1), and an enlarged liver and spleen can be seen (Figure 2). An ultrasound of the lymph nodes suggested large bilateral cervical, supraclavicular, submandibular, bilateral axillary, and bilateral inguinal lymph nodes with structural abnormalities. The gastroscopy results showed multiple longitudinal erosions and ulcers in the lower esophagus, multiple ulcers in the gastric sinus and horn (stage A1), chronic active fundus and corporitis, and distorted and deformed gastric sinus. No biopsy was taken because the patient had extremely low platelets.
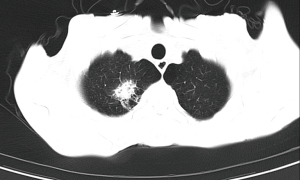
Based on the above test results and the patient’s recurrent fever, we considered the patient to be infected with AIDS combined with pulmonary infection, with the specific causative agent unknown. For the fever and hepatosplenomegaly, we could not exclude that it was due to immune system activation, such as hemophagocytic lymphohistiocytosis.
We empirically used antibacterial and antifungal as well as antiviral drugs (meropenem 1,000 mg q6h, voriconazole 200 mg q12h, ganciclovir 250 mg q12h) with no significant drop in the patient’s temperature (Figure 3). We took into account that AIDS is usually coinfected with tuberculosis, and although TB-IGRA was repeatedly negative, diagnostic antituberculosis treatment was added; however, the patient’s temperature still did not drop (Figure 3). During the empirical treatment, we performed a bone marrow aspiration, but the pathology department did not report any significant abnormalities. Due to the low elevation of triglyceride and ferritin and the absence of typical hemophagocytic cells in bone marrow examination, the diagnosis of hemophagocytic syndrome is not considered for the time being. As the patient’s platelet count was too low, many invasive tests were limited, such as lymph node puncture and fiberoptic bronchoscopy. The patient remained hyperthermic and did not respond to multiple medications, and we encountered difficulties in the diagnosis and treatment of the disease. Due to the patient’s HIV infection and low CD4 cell count, we still considered the cause of his fever as infection rather than tumor or immunogenic. After many considerations, we examined the patient’s blood using metagenomic next-generation sequencing (mNGS), which suggested Leishmania donovani (sequence number 9569) and Leishmania infantum (sequence number 6233).
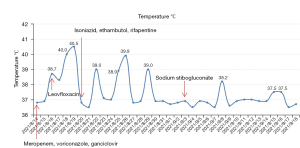
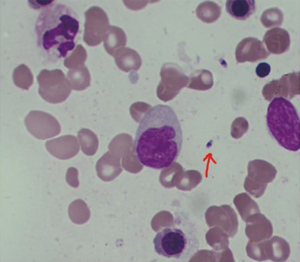
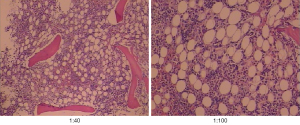
Then, the patient’s blood was sent to the Sichuan Center for Disease Control (CDC) for testing, which indicated that KR-39 was positive. After receiving the results, we discussed the case with the pathologist, and several pathology professors read the bone marrow smear and biopsy specimens again, and ultimately the Leishman-Donovan body (Figure 4) and tissue intracellular pathogens were found (Figure 5). All pathologists agreed on the diagnosis of Leishmania protozoa infection. At this point, we confirmed the diagnosis of VL based on the mNGS, rK-39, and bone marrow results. Later, sodium stibogluconate (SSG) (0.6 g qd) was added for treatment. The patient’s temperature decreased.
The patient subsequently had repeat CT and blood tests. There was a slight improvement in complete cytopenia (Table 2), and the imaging suggested an improvement in the lung infection (Figure 6) and a smaller liver and spleen than before. With stable symptoms, the patient was successfully discharged from the hospital.
Table 2
| Parameters | Reports | Comments |
|---|---|---|
| Hb | 83 g/L | Decreased |
| RBC count | 2.57×109/L | Decreased |
| TLC | 2.62×109/L | Decreased |
| Platelets | 42×109/L | Decreased |
Hb, hemoglobin; RBC, red blood cell; TLC, total leukocyte count.
After being discharged from the hospital, the patient was given anti-HIV treatment with tenofovir, lamivudine, and efavirenz. The patient had no significant discomfort and no increase in body temperature, his hemoglobin and platelets increased further 3 months after discharge (Table 3). He demonstrated a further reduction in liver and spleen size and a weight gain of 1.5 kg.
Table 3
| Parameters | Reports | Comments |
|---|---|---|
| Hb | 114 g/L | Decreased |
| RBC count | 3.42×109/L | Decreased |
| TLC | 1.88×109/L | Decreased |
| Platelets | 57×109/L | Decreased |
Hb, hemoglobin; RBC, red blood cell; TLC, total leukocyte count.
Literature review
Transmission
Female sandflies become infected if they suck the blood of infected human beings or mammals. Amastigotes transform in the sandfly gut and replicate as promastigotes; in about a week, the mature promastigotes will gather in the sandfly’s mouthparts. When the skin of a human or animal is penetrated, the promastigotes will invade the bloodstream (5). Some of the promastigotes entering humans or mammals are destroyed by phagocytosis of the polymorphonuclear leukocytes, while the rest enter macrophages. After entering macrophages, promastigotes lose their flagella and are transformed into amastigotes. Leishmania not only survives in the vacuole but also divides and multiplies, which eventually leads to the rupture of macrophages. Amastigotes can be engulfed by other macrophages, and then the above proliferation process is repeated. Occasionally, transmission does not require an insect vector. VL can be initiated directly by amastigotes through blood (shared needles, blood transfusions, and placental transmission) or organ transplants. Transmission by blood is also more common in patients with coinfected HIV (6-8).
Pathogenesis
In the immune response to the Leishmania infection, the CD4+ helper T cells (Th) have a key role, in which Th1 cells promote cellular immunity by secreting IL-2, IFN-γ, and TNF-α, which may cause the infection to subside; Th2 cells promote humoral immunity by secreting IL-4, IL-5, IL-6, IL-10, and IL-13, which enhance the production of antibodies that are not immune potent against Leishmania and may contribute to the infection spread. Cytokines play an important role as mediators, with Th1 and Th2 cytokines acting antagonistically to each other, and which type prevails will determine the development and regression of the infection (9). As the time after Leishmania infection is prolonged, the antigens that stimulate the immune system also change, which can induce complex and multiple cytokine changes. It has been shown that Leishmania infection is accompanied not only by suppression of specific cellular immunity but also leads to a reduction in the body’s ability to produce cellular and humoral immune responses to other antigens, a response known as nonspecific immunosuppression. It can be seen that a large number of carriers of occult infections is the result of a balance between the pathogenicity of the protozoa and the immune protective properties of the organism (10). Macrophages and dendritic cells can be coinfected by Leishmania and HIV-1. Leishmania promotes a high secretion of TNF-α and IL-1α to enhance HIV-1 replication; Leishmania infantum promastigotes reduce HIV-1 replication in the early stage of growth and enhance HIV-1 replication as time progresses (11-13). Therefore, Leishmania and HIV-1 can cause complex interactions in their prevalent natural host cells.
When this balance is disturbed, the invisible infection ceases to exist and is replaced by a series of overt infections with varying manifestations.
Clinical manifestations
More than 20 well-recognized Leishmania species are known to infect humans. The clinical manifestations of leishmaniasis are influenced by the leishmanial species, the site of invasion, and the host’s immune status. Leishmaniasis infections can manifest as occult or subclinical forms, localized lesions, and diffuse infections and can be interconverted. They can be roughly divided into three categories: VL, cutaneous leishmaniasis (CL), and mucocutaneous leishmaniasis (ML) (5,14). VL, also called ‘kala-azar’ in China and South Asian countries, is usually caused by the Leishmania donovani or Leishmania infantum infection. According to observations of patients with VL in Southwest Europe, they generally have fever (87–88%), splenomegaly (74–78%), hepatomegaly (49–79%), and lymph node enlargement (50%), in addition to typical symptoms of VL such as leukopenia (79%), anemia, and weight loss (15). The clinical manifestations of HIV-coinfected patients are generally similar to those of VL caused by a single leishmanial infection. Without treatment, mortality is very high.
The main symptoms of CL are skin ulcers and permanent scarring on the exposed parts of the limbs. ML can be considered a special type of CL, mainly invading the mucous membrane of the nasopharynx and lips, producing ulcers that can cause deformities in the nasopharynx. The secretions from the lesions flowing into the trachea can cause aspiration pneumonia or airway obstruction leading to death.
Epidemiology
More than 90% of VL cases globally occur in six countries: Bangladesh, Brazil, Ethiopia, India, South Sudan, and Sudan (16,17). There are two probable reasons for the high prevalence in these regions: firstly, geographically, their climate is suitable for the survival and growth of Leishmania and the insect vector, and secondly, the high prevalence is most likely related to economic development. Lower levels of economic development mean lower energy and nutritional intake in the population and also lower investment in health costs. War, famine, and displacement all contribute to significantly higher morbidity and mortality rates (18). In 88 countries and regions around the world, the number of patients is more than 12 million, the disability-adjusted life years (DALYs) is 2.357 million, the threatened population is about 350 million, and 2 million new cases are added every year, including 500,000 patients with VL and 1.5 million patients with CL (19). A study estimating the incidence of leishmaniasis globally suggested approximately 0.2–0.4 million and 0.7–1.2 million VL and CL cases, respectively, occur each year, and using a 10% overall mortality rate, gave a preliminary estimate of 20,000–40,000 leishmaniasis deaths per year (16). Because surveillance and vital record reporting in the countries most affected by leishmaniasis are incomplete and mortality data are very sparse—often representing only hospital deaths—the authors of this article also feel that the true incidence of leishmaniasis may be much higher and that mortality estimates are more uncertain than morbidity estimates.
In China, the annual incidence is now very low at only 0.03/100,000, according to the available 2011 official record (20). VL occasionally occurs in central and western areas of China, such as Shanxi, Shaanxi, Sichuan, Gansu, Xinjiang, and Inner Mongolia.
Diagnosis
The diagnosis of all suspected Leishmania infections can be approached in three ways: clinical, immunologic, and pathogenic.
- Clinical diagnosis: the symptoms of Leishmania infection are as described above. They are not specific, and the clinical presentation is more varied in patients with coinfection. It is difficult to confirm the diagnosis by clinical presentation alone. Apart from a history of travel to leishmaniasis-affected areas during the sandfly season, intravenous drug use, unsafe sex practices, and other high-risk factors, confirming the diagnosis is still primarily based on experimental diagnosis.
- Immunologic diagnosis: the most widely used immunological test is the rapid test based on the rK39 antigen. K39 is a conserved sequence containing 39 amino acids isolated from Leishmania chagasi, and its gene fragment recombinant antigen is rK39. The test of rK39 has a high sensitivity (97–100%) and specificity (83–85%) for the diagnosis of VL (20).
- Pathogenic diagnosis: the detection of Leishmania is the gold standard for the diagnosis of leishmaniasis. Microscopic examination of bone marrow, lymph node, and spleen aspirates is the most reliable method for confirming the diagnosis of VL. Spleen aspiration fluid has the highest diagnostic value (specificity and sensitivity >90%), followed by bone marrow (sensitivity 53–86%) and lymph nodes (sensitivity 53–65%). However, because most VL patients have significantly reduced platelet counts, the above invasive tests are risky and often difficult to complete.
Whether clinical, immunologic, or parasitological, diagnosis requires that the clinician has a sufficient amount of knowledge about the disease. As mentioned above, clinical workers in non-endemic areas may not have enough knowledge of the disease to perform specific tests (such as rK39). There is also the possibility of failure to identify the pathogen under microscopic examination, leading to misdiagnosis and underdiagnosis.
The mNGS test can be considered a pathogenic method that sequences DNA or RNA from clinical specimens and compares it to a database of characteristic sequences to identify pathogens. It can use samples directly obtained from a patient and amplify the sequences of all organisms in the sample, including host sequences. This unbiased approach allows for the detection of multiple types of pathogens in one sample. The advantages of it compared with traditional sequencing methods include higher throughput with sample multiplexing, higher sensitivity in detecting low-frequency variants, faster turnaround time for high sample volumes, and lower cost (21). As is known, infectious diseases remain one of the most important diseases to focus on globally (22). Identifying the pathogen and a clear diagnosis are of paramount importance, as misdiagnosis, missed diagnosis, and delayed diagnosis can lead to many adverse events, including unnecessary antibiotic use or misuse, increased medical costs, and deterioration in patient prognosis (23-25). Because mNGS has the above advantages, it is valuable for diagnosing infectious diseases. It is more time-sensitive and comprehensive than blood cultures, which take too long, or specific serologic tests. Although it cannot be used as a gold standard for definitive diagnosis, mNGS can play a supportive role in the diagnosis and give direction to clinicians. In our case, decision-making errors from both the clinician and pathologist caused some delays in correctly diagnosing the patient’s condition. This is a rare occurrence in large general hospitals in China, but it cannot be ruled out completely. In hospitals in China and other countries with much poorer diagnostic capabilities, the chances of missed diagnosis and misdiagnosis are much higher. Therefore, when economic conditions allow, mNGS may be chosen as a routine adjunctive test when multiple empirical treatments are ineffective and the pathogen cannot be identified.
Treatment
Currently, the commonly used efficacious agents for leishmaniasis include antimony and AMB. Other drugs, such as paromomycin and miltefosine, have shown to be not so effective, and some, although performing well in clinical trials, are still not in widespread use.
Antimony
In China, antimony is available in two preparations: SSG and meglumine antimoniate (MA). Both are chemically equivalent, with the active ingredient being the 5-valent antimony (26). The mechanism of action of pentavalent antimonial drugs is uncertain; the possible pharmacological mechanism is, in vivo, conversion to 3-valent antimony compounds that may be involved in both antileishmanial activity and drug toxicity (27). A previous study suggest that antileishmanial activity may occur via inhibition of parasite ADP phosphorylation, DNA I topoisomerase, or trypanothione reductase (28). In China, SSG has long been used as the first-line treatment for leishmaniasis, and the commonly used regimen is mainly the “six-day regimen”, while the “three-week regimen” can be used for patients who are in poor health or critical condition. Patients who are not cured after a course of treatment or relapse after being cured can consider increasing the dose and course of treatment. However, in Bihar, India, the incidence of the disease is high, comprising about 90% of cases in India and about 45% of cases worldwide. The local success rate for antimony treatment is only 35% (29). Leishmania can survive in macrophages for a long time, and antimony cannot completely kill the protozoa in the patient even after successful treatment. Especially for HIV-infected patients with reduced CD4+ lymphocytes, relapses usually occur within 3–6 months after treatment, and multiple relapses can cause VL symptoms to become atypical or remit, while relapses will become more frequent.
Also, antimony has numerous dose-related side effects, such as cardiotoxicity (manifested by T-wave inversion, Q-T interval prolongation, and various types of arrhythmias) and other adverse effects, including joint muscle pain, elevated liver enzymes, and pancreatic enzymes. Drug-induced prolongation of the Q-T interval >0.5 s significantly increases the risk of serious or fatal arrhythmias. Adverse drug reactions should be monitored closely during antimony administration, and ECG tests should be conducted regularly.
AMB
AMB is highly effective in treating VL, has a low recurrence rate, and remains effective after relapse with AMB treatment. The efficacy of AMB deoxycholate is confirmed, but the adverse effects are high. Liposomal AMB (L-AMB) enter macrophages more efficiently and induce side effects much less frequently than AMB deoxycholate. L-AMB is the only drug with US Food and Drug Administration (FDA) approval for VL treatment in the United States and, according to the World Health Organization (WHO), a total cumulative dose of 20 mg/kg is adequate to achieve high cure rates in immunocompetent patients with VL worldwide, regardless of the specific dosing schedule (14,30). For patients with impaired cellular immunity, the treatment failure rate and recurrence rate are particularly high, especially in HIV coinfection. In these cases, the usual strategy is to reuse L-AMB treatment because even after repeated treatment or preventive use, the parasite does not necessarily develop resistance to the drug (31,32).
The main adverse effects of AMB are nephrotoxicity, in addition to infusion reactions (thrombophlebitis), hypokalemia, myocarditis, leukocyte decline, and hepatic impairment. Therefore, AMB and L-AMB should only be administered in an inpatient setting at a specialist hospital, and the adverse effects should be closely observed during the treatment.
Discussion and conclusions
In Chengdu (the capital of Sichuan province), the incidence of VL is extremely low, so when our patient presented with fever, we did not initially consider leishmaniasis but rather common tuberculosis, bacterial, fungal infections, lymphoma, or other causes of hemophagocytic lymphohistiocytosis. The road to the correct diagnosis was full of twists and turns, and although we made the right diagnosis in the end, we could have done better. The patient was a middle-aged male working in Xinjiang, a population with a high prevalence of VL. This information was obtained during the history taking, but we did not pay enough attention to it. This suggests that clinical workers in non-infected areas are not sufficiently aware of the disease. For pathologists in non-endemic regions who do not have the relevant training or have never been involved in diagnosing this disease, there are also difficulties in recognizing Leishman-Donovan bodies in bone marrow smears, thus increasing the probability of missing the diagnosis. In large general hospitals in China, although this is an extremely rare occurrence, it cannot be completely avoided. However, in hospitals with lesser diagnostic capabilities, both at home and abroad, the probability of misdiagnosis and missed diagnoses is even greater. In the face of this potential risk, and if economic conditions allow, mNGS can be used as a routine test for patients in whom multiple empirical treatments have failed and the pathogen is unclear.
When Leishmania infection is detected, we can choose antimony and AMB as a treatment modality, while highly active antiretroviral therapy (HAART) is also essential for patients with coinfected HIV, targeting CD4+ >200 or even >400 cells/µL as an antiviral, which reduces the recurrence of leishmaniasis. The side effects of either antimony or AMB should not be ignored, and their use should be accompanied by long-term and frequent review of relevant indicators. Compared to both drugs, AMB has a higher clinical response rate and a lower relapse rate. Among the different preparations of AMB, L-AMB has a lower incidence of side effects. Regardless of the medication used, recurrence cannot be avoided entirely, so post-treatment follow-up is essential.
Today, with advanced means of transportation, hospitals in non-infected areas may still receive infected patients from infected areas, which is, of course, difficult to distinguish. Therefore, it is particularly important for clinicians to better understand the various diseases and to confirm the patient’s medical history and travel history.
The mNGS test can be considered a pathogenic method that sequences DNA or RNA from clinical specimens and compares it to a database of characteristic sequences to identify pathogens. It is more time-sensitive and comprehensive than blood cultures, which take too long, or specific serologic tests. Therefore, when economic conditions allow, mNGS may be chosen as a routine adjunctive test when multiple empirical treatments are ineffective and the pathogen cannot be identified. Leishmaniasis can be treated with antimony and AMB. L-AMB is highly recommended because of its better efficacy and lower side effects. In our case, although the patient’s temperature has not risen again, there is still a possibility of relapse, so we intend to continue with long-term follow-up.
Acknowledgments
We thank the West China Hospital of Sichuan University for providing the platform for this case report and the patient and his family for their contribution to the research.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1351/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1351/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sleasman JW, Goodenow MM. Pathogenesis and natural history of HIV infection. J Fla Med Assoc 1991;78:678-81. [PubMed]
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 2004;27:305-18. [Crossref] [PubMed]
- de la Loma A, Alvar J, Martinez Galiano E, et al. Leishmaniasis or AIDS? Trans R Soc Trop Med Hyg 1985;79:421-2. [Crossref] [PubMed]
- Leishmania/HIV co-infection, south-western Europe, 1990-1998. Wkly Epidemiol Rec 1999;74:365-75. [PubMed]
- Murray HW, Berman JD, Davies CR, et al. Advances in leishmaniasis. Lancet 2005;366:1561-77. [Crossref] [PubMed]
- Morillas-Marquez F, Martin-Sanchez J, Acedo-Sanchez C, et al. Leishmania infantum (Protozoa, kinetoplastida): transmission from infected patients to experimental animal under conditions that simulate needle-sharing. Exp Parasitol 2002;100:71-4. [Crossref] [PubMed]
- Pagliano P, Carannante N, Rossi M, et al. Visceral leishmaniasis in pregnancy: a case series and a systematic review of the literature. J Antimicrob Chemother 2005;55:229-33. [Crossref] [PubMed]
- Cruz I, Morales MA, Noguer I, et al. Leishmania in discarded syringes from intravenous drug users. Lancet 2002;359:1124-5. [Crossref] [PubMed]
- Ezra N, Ochoa MT, Craft N. Human immunodeficiency virus and leishmaniasis. J Glob Infect Dis 2010;2:248-57. [Crossref] [PubMed]
- Aebischer T. Recurrent cutaneous leishmaniasis: a role for persistent parasites? Parasitol Today 1994;10:25-8. [Crossref] [PubMed]
- Garg R, Barat C, Ouellet M, et al. Leishmania infantum amastigotes enhance HIV-1 production in cocultures of human dendritic cells and CD4 T cells by inducing secretion of IL-6 and TNF-alpha. PLoS Negl Trop Dis 2009;3:e441. [Crossref] [PubMed]
- Garg R, Lodge R, Descoteaux A, et al. Leishmania infantum promastigotes reduce entry of HIV-1 into macrophages through a lipophosphoglycan-mediated disruption of lipid rafts. J Infect Dis 2008;197:1701-8. [Crossref] [PubMed]
- Zhao C, Papadopoulou B, Tremblay MJ. Leishmania infantum promotes replication of HIV type 1 in human lymphoid tissue cultured ex vivo by inducing secretion of the proinflammatory cytokines TNF-alpha and IL-1 alpha. J Immunol 2004;172:3086-93. [Crossref] [PubMed]
- Georgiadou SP, Makaritsis KP, Dalekos GN. Leishmaniasis revisited: Current aspects on epidemiology, diagnosis and treatment. J Transl Int Med 2015;3:43-50. [Crossref] [PubMed]
- Rosenthal E, Marty P, le Fichoux Y, et al. Clinical manifestations of visceral leishmaniasis associated with HIV infection: a retrospective study of 91 French cases. Ann Trop Med Parasitol 2000;94:37-42. [Crossref] [PubMed]
- Alvar J, Vélez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 2012;7:e35671. [Crossref] [PubMed]
- Mathers CD, Ezzati M, Lopez AD. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl Trop Dis 2007;1:e114. [Crossref] [PubMed]
- Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends Parasitol 2006;22:552-7. [Crossref] [PubMed]
- Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg 2001;95:239-43. [Crossref] [PubMed]
- Lun ZR, Wu MS, Chen YF, et al. Visceral Leishmaniasis in China: an Endemic Disease under Control. Clin Microbiol Rev 2015;28:987-1004. [Crossref] [PubMed]
- Zhong Y, Xu F, Wu J, et al. Application of Next Generation Sequencing in Laboratory Medicine. Ann Lab Med 2021;41:25-43. [Crossref] [PubMed]
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. [Crossref] [PubMed]
- Ewig S, Torres A, Angeles Marcos M, et al. Factors associated with unknown aetiology in patients with community-acquired pneumonia. Eur Respir J 2002;20:1254-62. [Crossref] [PubMed]
- Karkhane M, Pourhosiengholi MA, Akbariyan Torkabad MR, et al. Annual Antibiotic Related Economic Burden of Healthcare Associated Infections; a Cross-Sectional Population Based Study. Iran J Pharm Res 2016;15:605-10. [PubMed]
- Weng QY, Raff AB, Cohen JM, et al. Costs and Consequences Associated With Misdiagnosed Lower Extremity Cellulitis. JAMA Dermatol 2017;153:141-6. [Crossref] [PubMed]
- den Boer M, Davidson RN. Treatment options for visceral leishmaniasis. Expert Rev Anti Infect Ther 2006;4:187-97. [Crossref] [PubMed]
- Frézard F, Demicheli C, Ribeiro RR. Pentavalent antimonials: new perspectives for old drugs. Molecules 2009;14:2317-36. [Crossref] [PubMed]
- Croft SL, Yardley V. Chemotherapy of leishmaniasis. Curr Pharm Des 2002;8:319-42. [Crossref] [PubMed]
- Sundar S, More DK, Singh MK, et al. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis 2000;31:1104-7. [Crossref] [PubMed]
- Bern C, Adler-Moore J, Berenguer J, et al. Liposomal amphotericin B for the treatment of visceral leishmaniasis. Clin Infect Dis 2006;43:917-24. [Crossref] [PubMed]
- van Griensven J, Carrillo E, López-Vélez R, et al. Leishmaniasis in immunosuppressed individuals. Clin Microbiol Infect 2014;20:286-99. [Crossref] [PubMed]
- Lachaud L, Bourgeois N, Plourde M, et al. Parasite susceptibility to amphotericin B in failures of treatment for visceral leishmaniasis in patients coinfected with HIV type 1 and Leishmania infantum. Clin Infect Dis 2009;48:e16-22. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)



