
Severe immune-related hepatitis and myocarditis caused by PD-1 inhibitors in the treatment of triple-negative breast cancer: a case report
Introduction
Triple-negative breast cancer (TNBC) is a special subtype of breast cancer. The expressions of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (Her-2) are all negative in TNBC; therefore, it does not respond to endocrine or targeted therapies. Compared with the other molecular subtypes of breast cancer, TNBC has the worst locoregional recurrence rates in the first 3 years after treatment. Furthermore, the incidence of distant metastasis and the 5-year mortality rate are also high. A previous study has shown that approximately 20% of TNBC patients present with programmed death ligand protein-1 (PD-L1) positivity and the PD-L1 mRNA expression is higher in TNBC compared with non-TNBC (P<0.001) (1). Adams et al. (2) confirmed that the objective response rate (ORR) of PD-L1 inhibitors combined with albumin paclitaxel in the treatment of metastatic TNBC was 66.7%. Thus, immune checkpoint inhibitors (ICIs) may be a breakthrough for the treatment of TNBC.
Programmed death-1 (PD-1) inhibitors help to restore the endogenous anti-tumor T cell response by blocking the binding of PD-1 to PD-L1 and PD-L2. TNBC is characterized by immune activation and infiltration. Among breast cancer subtypes, the expression of biomarkers associated with a response to immunotherapy is more common in TNBC (3). In 2020, the FDA accelerated the approval of the PD-1 inhibitors, pembrolizumab, combined with chemotherapy for locally recurrent, unresectable, metastatic PD-L1-positive TNBC. At present, clinical trials of PD-1 inhibitors for the treatment of advanced TNBC are underway in China (4). ICIs enhance the human immune response and cause immune system disorder, which may eventually damage normal human tissues and organs (5). Similar to inflammation or autoimmune diseases, these adverse events are defined as immune-related adverse events (irAEs). A greater number of irAEs have been reported recently owing to the widespread use of PD-1 inhibitors. In both the IMpassion 130 trial in the metastatic setting and Keynote 522 in the neoadjuvant setting, the most common grade 3–4 irAEs occurred in TNBC patients who received chemotherapy combined with PD-1/PD-L1 inhibitors were neutropenia, peripheral neuropathy, anemia, fatigue and pyrexia (6,7). The simultaneous odds of two severe irAEs are minimal. There have been a case report of two severe irAEs triggered by an anti-PD-1 ICIs in an advanced lung cancer patient (8), but no reports in TNBC patients. This article reports a case of immune-related hepatitis and immune-related myocarditis induced by PD-1 inhibitors in the treatment of metastatic TNBC, hoping to provide a reference for the prevention and treatment of irAEs. We present the following case in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1284/rc).
Case presentation
This clinical report concerns a 51-year-old woman treated at the First Affiliated Hospital of Soochow University in China (Figure 1). She has a history of contrast agent allergy and denies anamnesis of viral hepatitis, heart disease, or autoimmune disease. The patient underwent modified radical mastectomy for left breast ductal carcinoma, which was negative for ER, PR, and Her-2 on the 1st June 2016. She then received six cycles of postoperative adjuvant chemotherapy with paclitaxel liposome (175 mg/m2) and carboplatin [area under the curve (AUC) =5] (Figure 1A). No disease progression was observed during regular follow-up.
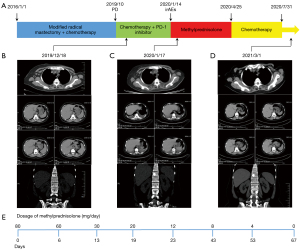
In October 2019, physical examination revealed lymphadenectasis in the right axilla. Puncture biopsy pathology of right axillary lymph node showed metastatic carcinoma, which tended to breast origin. The immunohistochemistry panel showed that the tumor cells were ER (−), PR (−), Her-2 (2+), and PD-L1 [combined positive score (CPS) =8]. Fluorescence in situ hybridization (FISH) detection of Her-2 was negative. Baseline computed tomography (CT) examination on the 18th December 2019 revealed multiple lymphadenectasis in the right axilla and pleural effusion (Figure 1B).
On the 19th December 2019, the patient received the first cycle of chemotherapy with albumin paclitaxel 200 mg on days 1 and 8, and cisplatin 60 mg on days 1 and 2 [every 3 weeks (Q3W)]. After alleviating the patient’s concerns and reservations regarding immunotherapy, the patient received a PD-1 inhibitor (200 mg) on the 3rd January 2020. Three days later, the patient experienced high fever, mild dyspnea, and systemic rash. Liver function indexes and cardiac markers also gradually increased (Figures 2,3). Laboratory examinations showed brain natriuretic peptide (BNP) 1,991 pg/mL on the 8th January 2020, and alanine aminotransferase (ALT) 2,611.8 U/L, aspartate aminotransferase (AST) 6,411.5 U/L, and total bilirubin (TBil) 70.5 µmol/L on the 14th January 2020. The patient underwent a CT examination on the 17th January, 2020 (Figure 1C), which showed little pericardial fluid and a new bone metastasis in the sternum, right fifth rib, right iliac bone, thoracic vertebras (T5, 8, 12), and lumbar vertebras (L1, 3). Also, the CT showed no hepatic or cardiac metastasis.
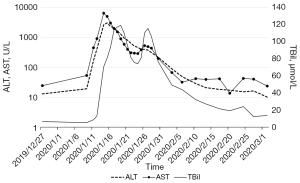
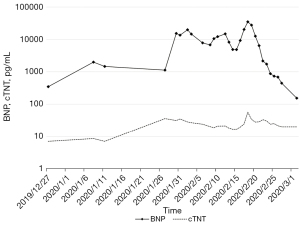
Based on the clinical manifestations and medical examinations, the patient was diagnosed with immune-related hepatitis and immune-related myocarditis induced by PD-1 inhibitors. We started with the intravenous administration of methylprednisolone (80 mg/day), antibiotics, and hepatic protectors on the 14th January 2020. The dose of methylprednisolone was reduced to 60 mg/day when ALT, AST, and TBil decreased to 945.3 U/L, 586.0 U/L, 109.9 µmol/L, respectively, on the 20th January 2020. After 13 days of continued treatment, the patient’s liver function improved and the dose of methylprednisolone was gradually reduced to 20 mg/day. Laboratory examination on the 6th February 2020 showed ALT 48.9 U/L, AST 31.8 U/L, and TBil 43.9 µmol/L. Methylprednisolone was discontinued and oral methylprednisolone tablets (12 mg/day) were given for 20 days. Laboratory examinations on the 26th February 2020 revealed that the myocardial injury markers and liver function indexes had returned to normal levels, and the patient’s clinical symptoms had improved. We reduced the dosage of oral methylprednisolone tablets to 8 mg/day, and gradually ceased the use of this drug (Figure 1E).
After recovering from the irAEs, the patient completed five cycles of chemotherapy with albumin paclitaxel and cisplatin from the 25th April 2020 to the 31st July 2020. It was suggested the patient under genetic testing to determine the reason for the irAEs and provide guidance for subsequent treatment. The molecular genetics screening results are shown in Table 1. As of 1st March 2021, regular follow-up CT examinations revealed stable disease (SD) (Figure 1D).
Table 1
| Items | Result |
|---|---|
| ALK | Positive |
| BRAF | Negative |
| BRCA1/2 | Negative |
| bMSI | MSS |
| EGFR | Negative |
| EPCAM | Negative |
| FGFR2/3 | Negative |
| HER2 | Positive |
| HRR | Negative |
| JAK1 | Positive |
| KEAP1 | Negative |
| KIT | Positive |
| KRAS | Negative |
| MET | Negative |
| MLH1 | Negative |
| MLH3 | Positive |
| MSH2 | Positive |
| MSH6 | Negative |
| MTOR | Positive |
| NARS | Negative |
| NTRK1 | Positive |
| NTRK2/3 | Negative |
| PDGFRA | Positive |
| PIK3CA | Negative |
| RET | Negative |
| ROS1 | Negative |
| STK11 | Positive |
MSS, microsatellite stable.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
This patient with metastatic TNBC developed recurrent high fever, as well as rashes to her extremities and face after receiving one cycle of paclitaxel-carboplatin (TP) chemotherapy combined with a PD-1 inhibitor. Laboratory examinations revealed that her liver transaminase and myocardial injury markers increased significantly. The patient denied anamnesis of viral hepatitis, heart disease, or autoimmune disease, and CT examination excluded hepatic or cardiac metastasis. The Naranjo Probability Scale for Adverse Drug Reactions was used to evaluate the patient’s condition: similar adverse events had been reported previously (1 point), occurred after the use of PD-1 inhibitors (2 points), excluding other possible causes (2 points), existing clinical symptoms and abnormal laboratory examination results (1 point), and the patient was relieved after drug withdrawal and treatment (1 point). Our patient had a Naranjo Probability Scale score of 7 points, so the irAEs were likely caused by the PD-1 inhibitors treatment.
Immune-related hepatitis can occur at any time after immunotherapy, and mainly manifests as an increase in ALT and/or AST, with or without an increase in TBil. Immune-related hepatitis typically has no characteristic clinical symptoms, and occasionally causes fever, fatigue, loss of appetite, or other non-specific symptoms (9). Increased TBil can result in yellow staining of skin and sclera and yellowish urine. Immune-related myocarditis mainly manifests as some non-specific clinical symptoms such as fatigue, and it can also appear as specific cardiovascular disease symptoms or signs, such as acute heart failure and arrhythmia. Immune-related myocarditis is often accompanied by an increase in serum myocardial injury markers; troponin is more specific, and BNP is mainly used as an indicator of acute myocardial injury, such as left ventricular dysfunction. Echocardiography displays diffuse left ventricular systolic function, intracardiac changes, and local wall motion abnormalities. However, left ventricular systolic dysfunction caused by immune-related cardiotoxicity is generally mild or normal, and a normal ventricular ejection fraction cannot exclude the diagnosis of immune-related myocarditis (10). Myocardial biopsy or histopathology are the gold standard criteria for the diagnosis of immune-related myocarditis (11). Pathological results are often difficult to obtain in actual clinical work, so immune-related myocarditis can be diagnosed by combining myocardial enzymes, imaging examinations, and clinical symptoms.
According to the NCCN adverse event evaluation criteria, our patient’s immune-related hepatitis and immune-related myocarditis were defined as grade 4. In such cases, immunotherapy must be permanently stopped, glucocorticoid therapy should be started at 1–2 mg/(kg·d) prednisone/methylprednisolone, and continuous electrocardiogram (ECG) monitoring and daily retesting of liver function are also necessary. After 3 days of treatment, if the symptoms are improved, the glucocorticoid dose can be gradually reduced until the liver transaminases recover to below three times upper limit of normal (ULN) and cardiac function returns to baseline, and the reduction has to last for at least 1 month. If glucocorticoid resistance occurs or there is no improvement after 3 days, other immunosuppressants, such as mycophenolate mofetil, can be considered. Bao et al. (8) presented a case report about an advanced non-small cell lung cancer patient received a PD-1 inhibitors combined with chemotherapy and experienced severe hepatitis and pneumonitis. Systemic glucocorticoids were used according to the consensus after diagnosis of irAEs. Liver function and oxygenation improved gradually and the dosage of glucocorticoids was adjusted. Approximately 8 weeks later, the patient recovered from immune-related hepatitis and interstitial pulmonary changes were absorbed. Compared with the case reported by Bao et al. (8), the types of cancer and irAEs were different, however the patient’s liver function and cardiac function improved after the use of glucocorticoids in this case.
For our patient, after one cycle of chemotherapy combined with immunotherapy, CT displayed multiple new bone metastases, and the efficacy was evaluated as progressive disease (PD). Immunotherapy was stopped due to severe irAEs. After recovering from the irAEs, five cycles of TP chemotherapy were completed. Follow-up CT showed that the metastatic foci were similar to those previously, and the curative effect was evaluated as SD. Therefore, the post-immunotherapy bone metastases was more likely to indicate pseudoprogression. Pseudoprogression is the increase in tumor volume or the number of lesions after immunotherapy, followed by rapid tumor shrinkage or SD, and usually occurs after the initial immunotherapy (12). Pseudoprogression may be due to the activation of tumor immunity leading to increased infiltration of tumor-infiltrating lymphocytes (TILs) (13). However, pseudoprogression and hyperprogressive disease (HPD) are difficult to distinguish in imaging examination, and HPD is defined as tumor growth ratio (TGR) or tumor growth kinetics (TGK) >2 compared with that before using ICIs therapy (14).
The irAEs caused by ICIs may have been related to the non-specific activation of the immune system, reducing autoimmune tolerance and breaking the immune balance. However, the specific mechanism remains unclear. Currently, there are three hypotheses that explain the occurrence and development of irAEs (15): (I) ICIs induce downstream immune responses by binding to target receptors in non-tumor tissues; (II) effector T cells induce immune attack by reactivating antigens in non-target tissues; and (III) ICIs induce the production of inflammatory factors. An increasing number of irAEs have been reported owing to the widespread use of ICIs in oncotherapy, and the related predictive markers have become a research hotspot. It has been found that intestinal flora (16), gene mutation polymorphisms (17,18), autoantigen antibodies (19,20), and cytokines released by immune cells (21,22) can predict irAEs. Furthermore, a previous study has reported that people with STK11 or KEAP1 mutations are resistant to immunotherapy (23). Our patient’s genetic test revealed a STK11 mutation, which predicted irAEs following the application of immunotherapy. However, mutations in STK11 or KEAP1 are not predictive indicators of efficacy, and STK11 or KEAP1 cannot currently be used as predictive genes for irAEs. More clinical studies are needed to confirm whether immune-negative genes can predict irAEs or the curative effect of ICIs.
TNBC has high expression of PD-L1 and TILs compared with other subtypes of breast cancer, which suggests TNBC may benefit from ICIs (1,24). A previous study has confirmed that taxane predicted increased T-cell blastogenesis and NK cell cytotoxicity in stage II/III breast cancer patients (25). A phase 3 IMpassion130 study suggests a clinically meaningful overall survival benefit with atezolizumab (PD-L1 inhibitors) plus nab-paclitaxel in PD-L1-positive TNBC (7). In this case, the patient suspended immunotherapy due to severe irAEs after only receiving the first cycle of TP chemotherapy combined with PD-1 inhibitors, however the duration of SD was more than 1 year. It suggests that patient may still be benefit from immunotherapy. The insufficiency is that we did not test negative predictive biomarker of immunotherapy before using PD-1 inhibitors for this patient. Although negative predictive biomarker of immunotherapy cannot predict the occurrence of irAEs at present, it can also remind clinicians to choose immunotherapy more carefully for patients with negative predictive biomarker of immunotherapy.
ICIs provide long-term survival for cancer patients and achieve significant curative effects in the field of oncology. In general, the incidence of irAEs in ICIs therapy is lower than that of chemotherapy. Also, most irAEs can be cured by suspension and glucocorticoids treatment, and the core principles of irAEs treatment are early prevention, early diagnosis, and appropriate management. Patients with older age, poor physical condition, and high tumor load are more likely to have a high incidence of irAEs and poor therapeutic effects; therefore, ICIs should be carefully selected for these patients. However, there is no accurate predictive marker of irAEs, and observing the variation in the patients’ conditions is crucial when using ICIs.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1284/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1284/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2014;2:361-70. [Crossref] [PubMed]
- Adams S, Diamond JR, Hamilton EP, et al. Phase Ib trial of atezolizumab in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer (mTNBC). J Clin Oncol 2016;34:abstr 1009.
- Heeke AL, Tan AR. Checkpoint inhibitor therapy for metastatic triple-negative breast cancer. Cancer Metastasis Rev 2021;40:537-47. [Crossref] [PubMed]
- Zhu Y, Zhu X, Tang C, et al. Progress and challenges of immunotherapy in triple-negative breast cancer. Biochim Biophys Acta Rev Cancer 2021;1876:188593. [Crossref] [PubMed]
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714-68. [Crossref] [PubMed]
- Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020;382:810-21. [Crossref] [PubMed]
- Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44-59. [Crossref] [PubMed]
- Bao Z, Sun X, Chen W, et al. Two severe adverse events triggered by an anti-PD-1 immune checkpoint inhibitor in an advanced lung cancer patient: a case report and review of the literature. Ann Transl Med 2021;9:1358. [Crossref] [PubMed]
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375-91. [Crossref] [PubMed]
- Palaskas N, Lopez-Mattei J, Durand JB, et al. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J Am Heart Assoc 2020;9:e013757. [Crossref] [PubMed]
- Tajiri K, Aonuma K, Sekine I. Immune checkpoint inhibitor-related myocarditis. Jpn J Clin Oncol 2018;48:7-12. [Crossref] [PubMed]
- Imafuku K, Hata H, Kitamura S, et al. Ultrasonographic findings can identify 'pseudoprogression' under nivolumab therapy. Br J Dermatol 2017;177:1726-31. [Crossref] [PubMed]
- Nishino M, Ramaiya NH, Hatabu H, et al. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol 2017;14:655-68. [Crossref] [PubMed]
- Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin Cancer Res 2017;23:4242-50. [Crossref] [PubMed]
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378:158-68. [Crossref] [PubMed]
- Perret RE, Josselin N, Knol AC, et al. Histopathological aspects of cutaneous erythematous-papular eruptions induced by immune checkpoint inhibitors for the treatment of metastatic melanoma. Int J Dermatol 2017;56:527-33. [Crossref] [PubMed]
- Meng Q, Guo H, Hou S, et al. Lack of an association of PD-1 and its ligand genes with Behcet's disease in a Chinese Han population. PLoS One 2011;6:e25345. [Crossref] [PubMed]
- Wang K, Zhu Q, Lu Y, et al. CTLA-4 +49 G/A Polymorphism Confers Autoimmune Disease Risk: An Updated Meta-Analysis. Genet Test Mol Biomarkers 2017;21:222-7. [Crossref] [PubMed]
- Da Gama Duarte J, Parakh S, Andrews MC, et al. Autoantibodies May Predict Immune-Related Toxicity: Results from a Phase I Study of Intralesional Bacillus Calmette-Guérin followed by Ipilimumab in Patients with Advanced Metastatic Melanoma. Front Immunol 2018;9:411. [Crossref] [PubMed]
- Kobayashi T, Iwama S, Yasuda Y, et al. Patients With Antithyroid Antibodies Are Prone To Develop Destructive Thyroiditis by Nivolumab: A Prospective Study. J Endocr Soc 2018;2:241-51. [Crossref] [PubMed]
- Matsuo N, Azuma K, Hattori S, et al. Association between soluble immune mediators and tumor responses in patients with nonsmall cell lung cancer treated with anti-PD-1 inhibitor. Int J Cancer 2019;144:1170-9. [Crossref] [PubMed]
- Tarhini AA, Zahoor H, Lin Y, et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer 2015;3:39. [Crossref] [PubMed]
- Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov 2018;8:822-35. [Crossref] [PubMed]
- Kitano A, Ono M, Yoshida M, et al. Tumour-infiltrating lymphocytes are correlated with higher expression levels of PD-1 and PD-L1 in early breast cancer. ESMO Open 2017;2:e000150. [Crossref] [PubMed]
- Carson WE 3rd, Shapiro CL, Crespin TR, et al. Cellular immunity in breast cancer patients completing taxane treatment. Clin Cancer Res 2004;10:3401-9. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


