
Neoadjuvant radiotherapy for soft tissue sarcoma in China: a preliminary result
Introduction
Soft tissue sarcomas (STSs) are a group of malignant tumors derived from non-epithelial extraosseous tissues, but do not include the reticuloendothelial system, the neuroglia or the supporting tissues of various parenchymal organs (1). It is a group of mesenchymal tumors with a highly heterogeneous pathology, and an annual incidence of about 3.4/100,000 in the USA (2), and about 2.38/100,000 in China (3). Surgery plays an important role in the treatment of STS, and is not only the means to cure the disease, but also to obtain a pathological diagnosis (4,5). Combined with chemotherapy, radiotherapy (RT) and other comprehensive treatment measures, the overall 5-year survival rate is about 50–60% (6,7).
In recent years, with the development of targeted therapy, the efficacy of non-surgical therapy for certain types of sarcoma (e.g., gastrointestinal stromal tumors) has greatly improved, but the role of chemotherapy in the treatment of STS is still very limited. Therefore, comprehensive treatment mainly refers to combined RT and surgery. Radiation therapy for tumors is a local treatment that uses radiation therapy tumors. In recent years, intensity-modulated RT (IMRT) has become widely used globally, because it can maximize the dose for the tumor, reduce the dose to the surrounding normal tissue, reduce the irradiation volume, and thus reduce the complications, but also improve the local control rate of the tumor. It has been successfully used in the treatment of STS (8-10).
RT for STS includes neoadjuvant RT (or preoperative RT), adjuvant RT (or postoperative RT), and palliative RT. The indications of neoadjuvant RT are: (I) high-grade STS: regardless of tumor size and location (11); (II) low-grade malignant STS: although controversial, RT is recommended for those with T2 stage tumor >5 cm or margin positive (12); (III) STS involving peripheral blood vessels or nerves. Compared with adjuvant RT, neoadjuvant RT shows almost no difference in the prevention of distant metastasis (13). The main advantages of neoadjuvant RT are tumor reduction, local improvement, and the possibility of respectability for inoperable patients (14,15). At the same time, the scope of operation tends to be reduced, which can better preserve postoperative function, eliminate micro-cancer nests and subclinical lesions, reduce tumor cell viability and the probability of local implantation and distant metastasis, and indicate sensitivity to chemotherapy. The delayed toxicity rate of preoperative RT is low, the blood supply of the preoperative tumor is abundant and thus sensitivity to RT is high, and the radiation field and dose can be lower than for postoperative RT (50 vs. 60–70 Gy). However, the major disadvantage of neoadjuvant RT compared to adjuvant RT is a significant increase in wound complications (35% vs. 17%) (13). Surgery is delayed and distant metastasis may occur during neoadjuvant RT. The surrounding tissue fibrosis after preoperative RT can make the surgical operation more difficult. Therefore, RT should be considered individually in the comprehensive treatment of STS, and factors such as the wound, tumor grade and resection margin should be taken into account.
Despite neoadjuvant RT for STS being used worldwide, it is rarely reported in China. In order to evaluate the efficacy and safety of neoadjuvant RT for STS, we conducted exploratory research of a multidisciplinary treatment model for STS in the Department of Orthopaedic Oncology Surgery, Beijing Ji Shui Tan Hospital, and the Department of Radiotherapy, Cancer Hospital Chinese Academy of Medical Sciences. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-98/rc).
Methods
Patient selection
Patients with confirmed advanced STS at Beijing Ji Shui Tan Hospital, who were considered as tumor boundary not clear, close to blood vessels and nerves, maximum diameter of tumor ≥2 cm, no distant metastasis and no contraindication of RT [no previous history of limb RT, Karnofsky Performance Scale (KPS) score >70, white blood cells >3.5×109/L, neutrophils >1.5×109/L, platelets >100×109/L]. Patients with rhabdomyosarcoma, primary neuroectodermal tumors, chondrosarcomas, osteosarcomas, or with distant metastases were excluded from the study. The histological diagnosis was based on the WHO classification (16).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Beijing Ji Shui Tan Hospital (No. 20220306) and the ethics committee exempts patients from informed consent.
Treatment plan
Neoadjuvant RT
Patients were referred to the Department of Radiotherapy, Cancer Hospital Chinese Academy of Medical Sciences and given conventional fractionated neoadjuvant RT [95% planning target volume (PTV) 50 Gy/2 Gy/25 f] for 5 weeks, such like Dr. Rosenberg (5).
Adverse reactions and imaging evaluation of neoadjuvant RT
Acute complications after neoadjuvant RT were evaluated with the acute scoring criteria of the European Organization for Research and Treatment of Cancer/Radiation Therapy Oncology Group (17). Late radiation toxicity according to the RTOG-0630 trial was followed up (18). At 4 weeks after RT, all patients were evaluated for local and distant metastases by magnetic resonance imaging/computed tomography (CT)/ultrasonography, and positron emission tomography-CT if possible. Each patient was classified as complete remission (CR), partial remission (PR), stable disease (SD), or progressive disease (PD) according to the response criteria of RECIST 1.1 (19). The objective response rate (ORR) was calculated by CR and PR, and the disease control rate (DCR) was calculated by CR, PR, and SD.
Surgery
Standard surgical excision was performed within 3–6 weeks after the end of RT. R0 excision was the main procedure, and some patients required a skin graft or vascular graft. Regarding the time interval between the end of RT and the operation, although somewhat controversial, 3–6 weeks is usually recommended (13,20), but there are reports of delaying for 8 weeks (18). The given interval is too short to achieve the goal of tumor reduction and reduced stage, and the tumor tissue was obviously necrotic and fibrotic, which shows the effect of RT, but at the same time, it is difficult to confirm the tumor’s boundary because of the surrounding hyperemia and edema. However, if the time interval is too long, then tumor regeneration and further delay of the operation may occur (21,22).
Surgical wound complications
All cases were examined by the team (including nurses, surgeons, and oncologists) to assess and evaluate wound complications in accordance with the criteria used by O’Sullivan et al. (13). Severe wound complications were defined as requiring “secondary surgery performed under general or local anesthesia for wound repair or management”.
Postoperative pathological evaluation
The pathological reaction was examined by a STS pathologist who was unaware of the tumor results, to determine whether pathological CR (pCR) was achieved. The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) histopathological response score (23) was used for pathological evaluation of patients after neoadjuvant RT. The method calculates the percentage of residual tumor cells and response is divided into 5 grades: A = no stainable tumor cells; B = single stainable tumor cells or small clusters (overall <1%); C =1% to <10% stainable tumor cells; D =10% to <50% stainable tumor cells; E =>50% stainable tumor cells. Patients with grade A by EORTC-STBSG were pCR.
Follow-up
Defined as the time interval between the date of surgery and the date of death or the date of the last follow-up. The no local recurrence interval was calculated from the date of operation. The patients were followed up every 3 months in the first 2 years, every 4 months in the next 3–4 years, every 6 months in the 5th year, and every year after 5 years. Follow-up included evaluating local recurrence and distant metastasis, disease-free survival (DFS), and long-term complications such as skin lesions, muscle stiffness, and limited mobility.
Statistical analysis
Complications of neoadjuvant RT, postoperative wound complications and pCR were evaluated after operation. SPSS23.0 statistical software was used and the Chi-square or Fisher accurate probability test was used to compare the rate of the two classifiers. P<0.05 was defined as statistical difference.
Results
Clinical features
From March 2017 to March 2018, a total of 19 patients were enrolled in the study (Table 1): the median age was 48 years (12–79 years), with 73.7% males (14/19) and 26.3% females. There are 17 cases (89.5%) of STS in the limbs and 2 cases (10.5%) in the pelvis (perineum and hip). The median tumor size was 7.2 cm (2–17.9 cm). Most cases were high-grade STS (84.2%), all of which were located in the deep fascia (100%). Liposarcoma (26.3%) was the most common histological subtype. Before RT, 3 cases (25.8%) were recurrent and 16 cases (84.2%) were primary.
Table 1
| Characteristics | Cases (n=19) |
|---|---|
| Sex | |
| Male | 14 |
| Female | 5 |
| Age (years), median [range] | 48 [12–79] |
| Time interval between end of RT and operation (weeks), median [range] | 5.3 [3.4–8.0] |
| Site of primary tumor | |
| Upper limb | 3 |
| Lower limb | 14 |
| Pelvis (perineum + buttocks) | 2 |
| Pathologic type | |
| STS unclassified | 7 |
| Liposarcoma (pleomorphic, myxoid, dedifferentiated) | 5 |
| Synovial sarcoma, pleomorphic undifferentiated sarcoma, leiomyosarcoma, fibrosarcoma, acinar sarcoma, malignant myofibroblastic tumor, clear cell chondrosarcoma | 7 (1 case each) |
| Tumor status before neoadjuvant RT | |
| Primary | 16 |
| Recrudescence | 3 |
| Tumor size (cm) before neoadjuvant RT | |
| <5 | 5 |
| ≥5 | 14 |
RT, radiotherapy; STS, soft tissue sarcoma.
Toxicity of RT
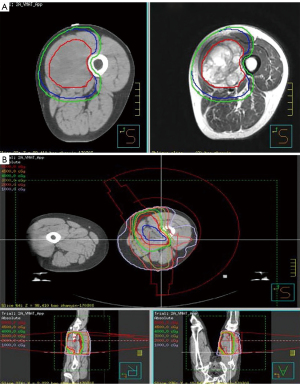
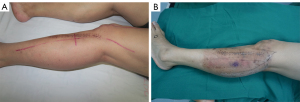
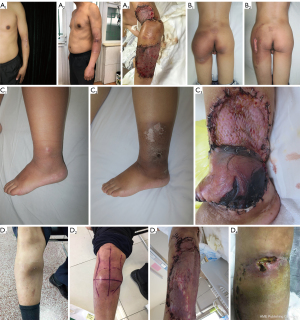
The usual dose of neoadjuvant RT was 50 Gy (2 Gy/day) for 5 weeks (14). The target area included the whole tumor body and the tumor periphery may invade the subclinical focus scope, the actual treatment range also includes offsets during routine treatment and uncertainties in patient positioning (Figure 1). Compared to RT for other tumors, the most common toxic reaction after neoadjuvant RT for STS is an acute skin reaction (Figures 2,3). In this study, the incidence was 68.4% (13/19), of which 7 cases (36.8%) were grade 1 and 6 cases (31.6%) were grade 2. All cases of skin toxicity recovered before operation.
Imaging evaluation after neoadjuvant RT
We used the standardized RECIST 1.1 criteria to evaluate the efficacy of neoadjuvant RT for STS. The main limitation of this method is that the evaluation of the primary lesion is one-dimensional and the size of local tumor may not change after RT. However, this standard is widely used in oncology and is easy to implement and explain, so we used it in this study. Of the 19 patients, 2 (10.5%) had PD (1 leiomyosarcoma and 1 pleomorphic liposarcoma), 6 (31.6%) had PR (1 pleomorphic liposarcoma, 1 undifferentiated pleomorphic sarcoma, 1 myxoid fibrosarcoma, 1 synovial sarcoma, 2 unclassifiable STS; 4 cases in the thigh, 1 case in the forearm, 1 case in the buttocks); 11 (57.9%) had SD; there were no cases of CR in this study. The ORR was 31.6% (6/19), and the DCR was 89.5% (17/19).
Surgery and surgical wound complications
All patients (100%) underwent limb salvage surgery with R0 resection. The median time between the end of neoadjuvant RT and the day of surgery was 5.3 weeks (range, 3.4–8.0 weeks). Postoperative wound complications occurred in 6 patients (31.6%) (Figure 3): 3 cases of prolonged wound healing time, which were relieved by intermittent dressing change, and 3 cases (15.8%) of severe complications (2 cases of flap necrosis and 1 local hematoma); all wounds healed well after secondary operation. The incidence of acute complications after neoadjuvant RT for STS has been reported as 30–43% in the literature (13,23), and the incidence in this study was within this range. Age (<60, ≥60 years old), the site of RT (upper limb, lower limb), the operation mode (local excision, local excision plus skin flap), the time interval between RT and operation (<5, ≥5 weeks), whether there was skin toxicity after RT, and the size of tumor (<5, ≥5 cm) were analyzed. There was no significant correlation between age, tumor site, time interval between RT and operation, skin toxicity after RT, skin flap transplantation, tumor size and wound complications (P>0.05). Late complications such as edema, joint stiffness, fracture, and skin fibrosis after RT were not observed in this study.
Postoperative pathological evaluation
There were 9 cases (9/19, 47.4%) of pCR. Considering the results of imaging evaluation, 83.3% of patients with PR after neoadjuvant RT were most likely to obtain pCR (Table 2; P<0.02), but we also found that pCR was possible even in PD patients, as occurred in 27.3% of SD patients. For the 10 patients who did not reach pCR, the pathologist in our center used the EORTC-STBSG histopathological response score for further assessment (15,23). Of the other patients underwent further assessment: there was 2 case of grade C (1% to <10% stainable tumor cells), 6 cases of grade D (10% to <50% stainable tumor cells), and 2 case of grade E (50% of stainable tumor cells).
Table 2
| Pathologic remissions | Cases, n [%] | P value | ||
|---|---|---|---|---|
| PD | PR | SD | ||
| pCR | 1 [50] | 5 [83] | 3 [27] | 0.022 |
| Non-pCR | 1 [50] | 1 [17] | 8 [73] | |
| Total | 2 | 6 | 11 | |
pCR, pathologic complete remission; PD, progressive disease; PR, partial remission; SD, stable disease.
Follow-up oncology results
Chemotherapy is also important in the treatment of STS. Even though neoadjuvant chemotherapy is controversial, it is often considered for patients with deep tumors, tumor >5 cm and with higher pathologic grade (24-27). Of the 19 patients in this group, 17 were recommended for adjuvant chemotherapy regimens with anthracycline and ifosfamide. Finally, 11 patients received adjuvant chemotherapy, and the remaining 8 patients refused.
The median follow-up time was 14.3 months (range, 11.9–24.7 months) (Figure 4). No cases of recurrence were found in the whole group. The 1-year non-recurrence rate was 100%. Lung metastases occurred in 2 patients (10.5%) during follow-up; therefore, the 1-year DFS rate for all the patients was 94.7%, and the 2-year DFS was 89.5%. The survival curve of patients with different pathologic evaluation results after neoadjuvant RT is shown in Figure 5, but there needs to be a longer follow-up. One of the two patients was a 48-year-old man with pleomorphic liposarcoma of the right thigh, and he developed multiple lung metastases 15 months after operation. He was in a clinical trial and his evaluation of neoadjuvant RT was PD on imaging and non-pCR under pathologic examination. The other patient was a 23-year-old man with alveolar STS of the right thigh, who developed a solitary pulmonary metastasis 9 months after surgery. He underwent thoracoscopic surgery, then a 6-week course adjuvant anthracycline-based chemotherapy and is now in follow-up. The evaluation of neoadjuvant RT was SD on imaging and non-pCR under pathologic examination. The two patients with metastasis were both non-pCR. However, the EORTC-STBSG histopathological response score and the incidence of metastasis seems no significant correlation for STS with neoadjuvant RT (Table 3; P>0.05).
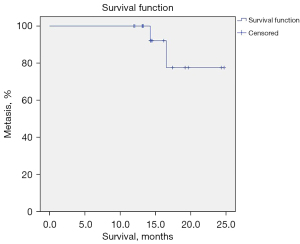
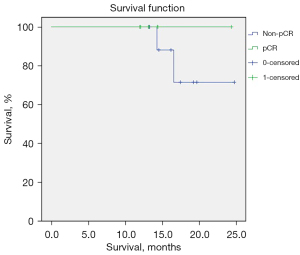
Table 3
| Pathologic remissions | Cases, n [%] | P value | |
|---|---|---|---|
| Metastasis | Non-metastasis | ||
| pCR | 0 | 9 [100] | 0.503 |
| Non-pCR | 2 [20] | 8 [80] | |
| Total | 2 | 17 | |
pCR, pathologic complete remission.
Discussion
The incidence of STS is relatively low, with a wide variety, wide distribution, and obvious heterogeneity. There are few clinical randomized controlled studies in China. Therefore, the best treatment for STS needs the participation of multiple disciplines. Surgical resection is the most important treatment method, and RT is a part of a multimodality strategy, which has been shown to reduce local recurrence in patients with STS in the extremities (23,28). Neoadjuvant RT is recommended for high-risk patients with the intention of increasing efficacy (29), and it is widely used abroad.
Advances in RT technology have improved its accuracy and reduced the damage to normal tissue around the lesion. IMRT has been found to reduce exposure to skin and solid tissue, and above all, reduce radiation-induced wound complications. A phase II clinical study in Canada confirmed that preoperative IMRT significantly reduced complications (including incision complications), minimized exposure to normal tissue, and maintained good limb function (29). This is a feasible, effective, and safe treatment for advanced STS of the limb (30,31), and 95% PTV 50 Gy/2 Gy/25 f is the currently recommended standard dose. High-level evidence is still lacking and the conduction of relevant clinical studies in qualified centers is recommended. Multidisciplinary cooperation of Departments of Orthopaedic Oncology Surgery, Radiotherapy, Oncology, Pathology, and other disciplines is a clinical model of great significance to improve the survival rate and functional status of patients. In neoadjuvant therapy, STSs are not sensitive to neoadjuvant chemotherapy, while neoadjuvant RT for STSs can improve the local control rate and preserve limb function. But in different centers in China, due to the different background of specialists and the different strengths of disciplines, the mode of multidisciplinary treatment is also different. This study was a cross-unit multidisciplinary collaboration, which made use of complementary advantages and a strong team working closely together.
The results showed that neoadjuvant RT was safe in STS. The most common side effect was mild grade 1–2 acute skin reaction (68.4%), and all cases of skin toxicity recovered before further operation. Wound complications occurred in 6 patients (31.6%) after surgery and could be controlled. There were severe complications in 3 cases (15.8%) and all patients underwent secondary surgery, then healed well. The local tumor DCR of neoadjuvant RT was as high as 89.5%, and the pCR rate was up to 47.4%.
There are some limitations to this study. Firstly, the number of cases was relatively small, due to the low incidence of STS and the strict indication of neoadjuvant RT. Because the national conditions in China, it is difficult to carry out a randomized controlled study of neoadjuvant RT and non-neoadjuvant RT in patients with STS. One of the important reasons is that Chinese surgeons have scruples about the wound complications after neoadjuvant RT. However, this single-center preliminary study has proved the feasibility and safety of neoadjuvant RT for STS in China, and given surgeons confidence to conduct follow-up studies. Secondly, the results were not inferior to another study with 15.7% recurrence rate and 15.7% metastasis rate (32), though the oncology results require a longer follow-up. Finally, the cost of neoadjuvant RT and the treatment of postoperative wound complications increased the patients’ medical expenses, but neoadjuvant RT is also within the scope of Medical Insurance Reimbursement in China.
In China, the effective cooperation of MDT between the department of RT and bone oncology should be improved, which can optimize the scientific radiation dose, target area and proper time interval. It’s necessary and helpful to improve the curative effect and reduce the complication of neoadjuvant RT.
Acknowledgments
We thank the multidisciplinary team of Orthopaedic Oncology Surgery, Pathology and Radiotherapy for their support in this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-98/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-98/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-98/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Beijing Ji Shui Tan Hospital (No. 20220306) and the ethics committee exempts patients from informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldblum JR, Weiss SW, Folpe AL. Enzinger and Weiss's soft tissue tumors. 6th ed. Philadelphia: Elsevier Health Sciences, 2013.
- Surveillance, Epidemiology, and End Results (SEER) Program. Based on the Dec 2018 data. Available online: https://seer.cancer.gov/
- Zheng R, Zhang S, Zeng H, et al. Incidence of soft tissue sarcoma in China. J Clin Oncol 2018;36:abstr e23560.
- Lehnhardt M, Hirche C, Daigeler A, et al. Soft tissue sarcoma of the upper extremities. Analysis of factors relevant for prognosis in 160 patients. Chirurg 2012;83:143-52. Erratum in: Chirurg 2012;83:485-6. [Crossref] [PubMed]
- Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982;196:305-15. [Crossref] [PubMed]
- Fletcher CDM, Unni KK, Mertens F. editors. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon: IARC, 2002.
- Kotilingam D, Lev DC, Lazar AJ, et al. Staging soft tissue sarcoma: evolution and change. CA Cancer J Clin 2006;56:282-91; quiz 314-5. [Crossref] [PubMed]
- Pirzkall A, Carol M, Lohr F, et al. Comparison of intensity-modulated radiotherapy with conventional conformal radiotherapy for complex-shaped tumors. Int J Radiat Oncol Biol Phys 2000;48:1371-80. [Crossref] [PubMed]
- Alektiar KM, Brennan MF, Singer S. Local control comparison of adjuvant brachytherapy to intensity-modulated radiotherapy in primary high-grade sarcoma of the extremity. Cancer 2011;117:3229-34. [Crossref] [PubMed]
- Alektiar KM, Brennan MF, Healey JH, et al. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol 2008;26:3440-4. [Crossref] [PubMed]
- Pisters PW, Pollock RE, Lewis VO, et al. Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg 2007;246:675-81; discussion 681-2. [Crossref] [PubMed]
- Kaushal A, Citrin D. The role of radiation therapy in the management of sarcomas. Surg Clin North Am 2008;88:629-46. viii. [Crossref] [PubMed]
- O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 2002;359:2235-41. [Crossref] [PubMed]
- Davis AM, O'Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 2005;75:48-53. [Crossref] [PubMed]
- de Vreeze RS, de Jong D, Haas RL, et al. Effectiveness of radiotherapy in myxoid sarcomas is associated with a dense vascular pattern. Int J Radiat Oncol Biol Phys 2008;72:1480-7. [Crossref] [PubMed]
- Fletcher CDM, Bridge JA, Hogendoorn P, et al. WHO Classification of Tumours of Soft Tissue and Bone. Lyon: IARC, 2013.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341-1346. [Crossref] [PubMed]
- Wang D, Zhang Q, Eisenberg BL, et al. Significant Reduction of Late Toxicities in Patients With Extremity Sarcoma Treated With Image-Guided Radiation Therapy to a Reduced Target Volume: Results of Radiation Therapy Oncology Group RTOG-0630 Trial. J Clin Oncol 2015;33:2231-8. [Crossref] [PubMed]
- Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer 2016;62:132-7. [Crossref] [PubMed]
- Cannon CP, Ballo MT, Zagars GK, et al. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer 2006;107:2455-61. [Crossref] [PubMed]
- Lefevre JH, Mineur L, Kotti S, et al. Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J Clin Oncol 2016;34:3773-80. [Crossref] [PubMed]
- Lim SB, Choi HS, Jeong SY, et al. Optimal surgery time after preoperative chemoradiotherapy for locally advanced rectal cancers. Ann Surg 2008;248:243-51. [Crossref] [PubMed]
- Wardelmann E, Haas RL, Bovée JV, et al. Evaluation of response after neoadjuvant treatment in soft tissue sarcomas; the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) recommendations for pathological examination and reporting. Eur J Cancer 2016;53:84-95. [Crossref] [PubMed]
- O'Sullivan B, Griffin AM, Dickie CI, et al. Phase 2 study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma. Cancer 2013;119:1878-84. [Crossref] [PubMed]
- Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol 2012;13:1045-54. [Crossref] [PubMed]
- Movva S, von Mehren M, Ross EA, et al. Patterns of Chemotherapy Administration in High-Risk Soft Tissue Sarcoma and Impact on Overall Survival. J Natl Compr Canc Netw 2015;13:1366-74. [Crossref] [PubMed]
- Italiano A, Delva F, Mathoulin-Pelissier S, et al. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: a multivariate analysis of the French Sarcoma Group Database. Ann Oncol 2010;21:2436-41. [Crossref] [PubMed]
- Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998;16:197-203. [Crossref] [PubMed]
- Sherman KL, Wayne JD, Chung J, et al. Assessment of multimodality therapy use for extremity sarcoma in the United States. J Surg Oncol 2014;109:395-404. [Crossref] [PubMed]
- Townley WA, Mah E, O'Neill AC, et al. Reconstruction of sarcoma defects following pre-operative radiation: free tissue transfer is safe and reliable. J Plast Reconstr Aesthet Surg 2013;66:1575-9. [Crossref] [PubMed]
- Zhou Y, Xie PM, Dong C, et al. Prospective clinical study of pre-operative SIB-IMRT in preparing surgical boundary of extremity soft tissue sarcoma. Eur Rev Med Pharmacol Sci 2015;19:4738-50. [PubMed]
- Peeken JC, Knie C, Kessel KA, et al. Neoadjuvant image-guided helical intensity modulated radiotherapy of extremity sarcomas - a single center experience. Radiat Oncol 2019;14:2. [Crossref] [PubMed]
(English Language Editor: K. Brown)


