
Intraoperative salvaging of failure to harvest the bi-paddle anterolateral thigh flap: a case series
Introduction
The anterolateral thigh (ALT) flap is currently one of the most popular workhorse flaps, due to its great versatility, availability of free amounts of tissue, minimal morbidity to the donor site, and preservation of the main artery (1,2). The bi-paddle flap is a special form of ALT flap that is defined as two or more perforator flaps harvested from the same donor area. Based on separate perforators, the ALT flap with two or more perforators can be split into two subunits, which in turn assists in the maintenance of relatively independent rotation, enabling the repair of irregular, large or complicated defects (3-5). Moreover, turning the length to width of the bi-paddle flap enables primary closure of the donor site. There are several reports regarding the clinical application of the bi-paddle flap, such as the bi-paddle radial artery flap (6), the double skin paddle ALT flap (7,8) and the bi-paddle thoracodorsal artery flap (9). It provides bespoke cover for large soft-tissue defects with improved morbidity and cosmesis of the donor site; more tissue can be harvested because of the multiple perforators (8).
Nonetheless, the ALT flap is characterized by variations in the source of blood vessels [the lateral circumflex femoral artery (LCFA) contained ascending branch, oblique branch, descending branch and transverse branch] and in the number of perforators (10,11), bringing great uncertainty to bi-paddle ALT free flap harvest. Kubo et al. reported that the lobulated flap was successfully harvested in only 50% of cases of through-and-through defects (12). In a study of 110 patients who underwent ALT flap operation, Lee et al. found that in 18.2% cases there was only one perforator, suggesting that in almost 1 out of 5 cases the patient’s anatomy is ineligible for a bi-paddle flap (13). Hence, dealing with flap harvesting failure remains a big challenge even for experienced micro-surgeons (3,12). This case series retrospectively analyzed 9 cases in which harvesting a bi-paddle flap failed because of patients’ anatomic variations, but was salvaged intraoperatively by converting the harvested free flap to another form immediately, according to our salvaging algorithm. We present our experience and explore the optimal algorithm to overcome harvesting failure and difficulties with the bi-paddle ALT flap. We present the following article in accordance with the AME Case Series reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1118/rc).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was a single-center consecutive case series analysis approved by the Ethics Committee of the 920th Hospital of Joint Logistics Support Force of Chinese PLA (the research registry No. E-R 2014-079-01). All patients in this study were informed that their medical information would be used for scientific purposes and they or their guardians gave written informed consent for publication of this study.
Surgical treatment with a bi-paddle ALT flap for soft-tissue defects of extremities was performed in 36 patients between 2014 and 2020 in 920th Hospital of Joint Logistics Support Force of Chinese PLA. However, in 9 cases there were difficulties or failure to harvest the flap. Therefore, inclusive criteria for this study were: (I) variations in the perforators of the ALT flap and (II) difficulties or failure to harvest the bi-paddle flap. All defects in the 9 patients ranged from 48 to 288 cm2 and underwent debridement and dressing change as stage I, and were repaired as stage II after 1–2 weeks of infection control. Preoperatively, Doppler mapping of the perforators determined the design of the bi-paddle ALT flap, but because of variations in the sources of the blood vessels and in the number of perforators discovered intraoperatively, the bi-paddle flap could not be elevated. Our conversion method was based on the characteristics of the defect, the number of perforators, and the vascular sources to harvest a free flap in another form for defect repair. The course of the primary disease ranged from 2 to 4 months, with an average of 2.8 months. The patients were informed by telephone to return to the hospital for follow-up monthly.
Data collection and analysis
Age, sex, cause of injury/disease, localization, associated injuries and type, size of defect, number of perforators, size of flap, conversion method, complications and follow-up (5–24 months, average period of 11.5 months) were extracted from the medical charts. The curative effect was evaluated according to clinical manifestations. Only descriptive statistics were used (Table 1).
Table 1
| No. | Age (years) | Gender | Cause of injury | Localization | Associated injuries | Type of defect | Size of defect | Perforators | Size of flap | Converted method | Complication |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | Male | Machine avulsion injury | Left foot | – | Single defect | 13 cm × 14 cm | One | 9 cm × 25 cm |
Widened single perforator flap | Scar hyperplasia in the flap donor site |
| 2 | 31 | Female | Machine penetrating injury | Right hand | 3nd to 5th tendons and bone exposure in the dorsum; index finger defect | Penetrating wound with two defects | Volar defect was 2×5 cm; dorsal defect was 5×7 cm | One | 6 cm × 14 cm |
Deepithelialized two-paddle flap | Localized infection in receiving area healed by dressing |
| 3 | 16 | Male | Traffic injury | Left foot | Necrosis of 1st to 4th toes | Single defect | 13 cm × 14 cm | Two perforators derived from different sources | 7 cm × 28 cm |
Sequential chimeric flap | Bulky flap healed by reduction |
| 4 | 43 | Male | Machine crush injury | Left forearm and hand | Defects of extensor muscle group and radial nerve | Two adjacent defects | Forearm defect was 7×25 cm; hand defect 8×9 cm | Two perforators derived from transverse and descending branch | 8 cm × 36 cm |
Combined transverse-and-descending branches flap | Receiving site infection healed by dressing |
| 5 | 48 | Male | Traffic injury | Left hand | Fractures of 3rd to 5th metacarpals | Single defect | 11 cm × 12 cm | One | 12 cm × 13 cm |
Widened single perforator flap; skin graft | Venous compromise healed by revision surgery |
| 6 | 36 | Male | Machine crush injury | Right ankle | Fracture of the medial malleolus | Single defect | 9 cm × 13 cm | One | 11 cm × 12 cm |
Widened single perforator flap | Bulky flap healed by reduction for 3 times |
| 7 | 14 | Male | Machine crush injury | Right leg | Rupture of anterior tibial tendon | Single defect | 12 cm × 13 cm | Two perforators derived from different sources | 7 cm × 26 cm | Sequential chimeric flap | Wound dehiscence healed by debridement and suturing |
| 8 | 31 | Male | Machine crush injury | Right hand | Fractures of the 2nd to 5th metacarpals | Single defect | 9 cm × 13 cm | One | 10 cm × 15 cm |
Widened single perforator flap; skin graft | Receiving area infection healed by dressing |
| 9 | 53 | Female | Mauled by a bear | Right hand | 2nd to 5th metacarpal fracture and bone exposure | Penetrating wound with two defects | Volar defect was 3×4 cm; dorsal defect was 6×8 cm | One | 4 cm × 12 cm |
Deepithelialized two-paddle flap | Localized infection in receiving area healed by dressing |
Surgical technique
The classic antegrade cutting method was used (1). The medial incision is made first and the flap is elevated suprafascially or subfascially. The deep fascia is stretched on both sides to expose the vastus lateralis and rectus femoris muscles. The septocutaneous perforator is well protected during this process. The connection between the rectus femoris and vastus intermedius muscles is separated, and the rectus femoris is stretched inward and forward to expose the branches of the LCFA. The perforator is especially protected during separation. Finally, the position, number, and the source of the flap perforators become clearly visible.
The conversion methods were as follows. A de-epithelialized bi-paddle flap was used if there was a single perforator to repair penetrating wounds with two adjacent defects. The flap length was extended moderately. After flap harvesting, the epidermis and the dermis were removed from the distal segment of the flap based on the size and the distance between the defects, to convert to a de-epithelialized flap with two paddles. The proximal and distal flaps were wound through the tunnel between defects. The donor site was sutured directly.
A widened-single-perforator flap was applied in cases of one perforator to repair the single defects. Briefly, the flap was redesigned based on the defect size. The flap width was increased and the length was decreased. The whole defect was repaired by widening the single-perforator flap with skin grafting to the donor site.
A combined-branches flap was created when two perforators were derived from the transverse and descending branches of the LCFA respectively. The incision was extended to the proximal and distal ends of the LCFA system as far as possible for harvesting the LCFA trunk containing the transverse-and-descending branches with their perforators. A bi-paddle flap with combined transverse-and-descending branches of the LCFA system can also be split into two subunits, which in turn assists in the maintenance of relatively independent rotation. During the operation, the femoral nerve branch was protected, and the blood supply of the rectus femoris was preserved. The flap donor site was directly sutured.
A sequential chimeric flap was redesigned when two perforators from different branches of the LCFA were far apart. The flap containing the two perforators from the descending and oblique branches respectively was elevated. The distal end of the descending branch was preserved for vascular anastomosis to the proximal end of the oblique branch to create a sequential chimeric flap, which in turn was used to cover large defects or two adjacent wounds. Primary closure of the donor site was then performed.
Results
Of the 9 patients included (7 male, 2 female; age range, 14–53 years), there were 6 with a machinery injury, 2 with a vehicular injury, and 1 was mauled by a bear. All defects in the extremities of these patients were large and irregular.
In 4 cases of a single defect (range, 10–12×13–15 cm) and a single perforator found intraoperatively, the bi-paddle ALT flap was converted to a widened single-perforator flap based on the characteristics of the defect. In 2 cases of a penetrating wound (volar defect 3×4/2×5 cm; dorsal defect 6×8/5×7 cm, respectively) and a single perforator the flap was converted to a de-epithelialized flap. In 2 cases of a single defect (12×13 cm/13×14 cm, respectively) and two perforators derived from different sources, the flap was converted to a sequential chimeric flap based on the number and source of the perforators. In the 1 case of two defects in the left forearm (7×25 cm) and dorsal hand (8×9 cm), the flap with two perforators derived from transverse and descending branches was converted to a combined transverse-and-descending branches flap. In 1 case of a venous crisis the lesion healed after revision surgery without any graft loss. Two cases of localized infections in the receiving area were cured by dressing changes. One case of wound dehiscence and one case of infection in the flap donor site were healed by debridement and suturing. Follow-up for 5–24 months (average, 11.5 months) revealed that all flaps survived. The anesthesia and operative extend the expected times.
Case reports
Case 1
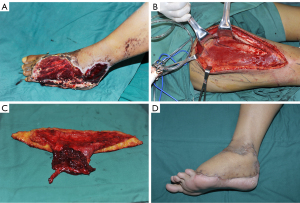
A 25-year-old male with a machine avulsion injury to the left foot had a 13×14 cm defect with extended tendon exposure and fracture of the 2nd–5th phalangeals. Preoperative design was a bi-paddle ALT flap from the left thigh to recover the large defect. A single perforator of the descending branch of lateral circumflex femoral artery (d-LCFA) was dissected intraoperatively, so the flap was harvested based on the variation of the perforator and the sizable defect and converted to a widened-single-perforator flap. The d-LCFA was anastomosed to the dorsalis pedis artery, and two concomitant veins to two recipient veins. The donor site was closed with a split-thickness skin graft harvested from the ipsilateral thigh. Fractures of the phalangeals were fixed by Kirschner-wire. The flap survived 10 days of hospitalization and at 5 months after transplantation, the site had a good-looking appearance (Figure 1).
Case 2
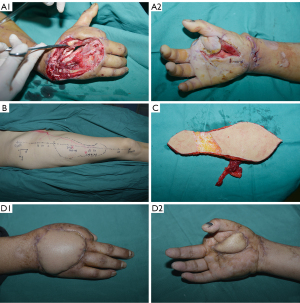
A 31-year-old female presented with a machinery penetrating injury to the right hand that had two adjacent defects: the dorsal defect was 5×7 cm with extended tendon and bone exposure; the volar defect was 2×5 cm and the index finger was absent. Preoperative design was a bi-paddle ALT flap from the left thigh to cover the two adjacent defects. Only one perforator of the d-LCFA was present intraoperatively, so based on this and the characteristics of the wound, salvage was performed by harvesting a de-epithelialized flap (6×14 cm) for wound repair. End-to-end anastomosis was performed between the d-LCFA and the right radial artery, and two concomitant veins with two recipient veins. The donor site required primary closure. The flap survived after 14 days of hospitalization, and at 1-year follow-up, it has a bulky appearance but the patient is satisfied (Figure 2).
Case 3
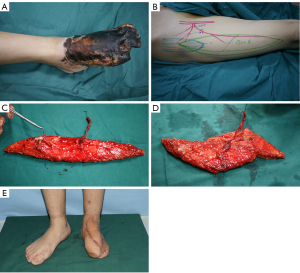
A vehicular injury to the left foot of a 16-year-old male was complicated by necrosis of the 1st–4th toes and skin of dorsal foot. The defect was 13×14 cm with exposure of the 1st and 2nd metatarsal bones and extended tendons after debridement and amputation of phalanges in stage I of treatment. A bi-paddle ALT flap from the right thigh was planned to repair the large defect. Intraoperatively, two perforators were derived from different source vessels (one perforator arising from the oblique branch of LCFA, another from the d-LCFA), making it impossible to harvest the paddles of the flap as a unit of a bi-paddle flap. The flap (7×28 cm) was harvested with an oblique branch paddle and a d-LCFA paddle and was split into two subunits. The distal run-off vessels of the d-LCFA were dissected for an enough length to anastomose them to the oblique branch to convert the flap to a sequential chimeric flap as a bi-paddle unit. The proximal d-LCFA were anastomosed in an end-to-end fashion to the left dorsalis pedis artery, and the two concomitant veins were anastomosed to the great saphenous vein and a recipient vein. After covering the bones, the residual wound was resurfaced using a split-thickness skin graft harvested from the left medial thigh. The flap and the skin graft survived with primary closure of the donor site after 10 days of hospitalization. Thinning of the flap was performed because of its bulky appearance after 4 months. The patient was satisfied with the appearance and function at final follow-up at 14 months (Figure 3).
Case 4
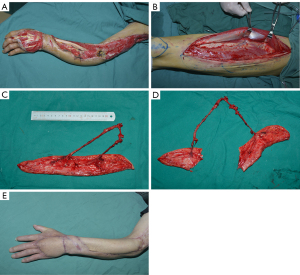
A 43-year-old male with a machinery crush injury presented with several soft-tissue defects in the left forearm and dorsal hand, defects of the extensor muscle group and radial nerve, and wounds on the dorsal forearm and dorsal hand (7×25 cm, 8×9 cm, respectively). The plan for a bi-paddle flap was halted by the finding of two perforators arising from the transverse and descending branches respectively, which originated from the same main vessel of the LCFA. The proximal end of the LCFA was dissected carefully to incorporate the transverse-and-descending branches as one unit and the flap was split to obtain a bi-paddle combined transverse-and-descending branches flap. The LCFA was anastomosed in an end-to-end fashion to the left radial artery. After covering the radius and finger extensor tendon, the residual wound of the lateral arm was resurfaced using a split-thickness skin graft harvested from the left medial thigh. The donor site infection was healed by debridement and suturing with primary closure. The flap survived after 20 days of hospitalization and at 5 months after transplantation, the bulky flap acquired a good-looking appearance after thinning (Figure 4).
Of the other 5 cases, 3 patients presenting with a single defect (ranging from 10–12×13–15 cm) and a single perforator found intraoperatively, the flaps were converted to widened-single-perforator flaps as in Case 1 based on the characteristics of defect. In 1 case of a penetrating wound (volar defect 3×4 cm; dorsal defect 6×8 cm), the flap was converted to a de-epithelialized flap as in Case 2. In the 5th case, a single defect (12×13 cm) and two perforators derived from different sources, led to the flap being converted to a sequential chimeric flap as in Case 3 based on the number and sources of the perforators.
Discussion
The most untoward aspect of the ALT flap is the well-documented complexity of the local vasculature, and clinical understanding of this variability cannot be overstated, as failure to do so can lead to vascular flap embarrassment and tissue loss (14). There are several variations in both the sources as well as the branches of the LCFA artery. The traditional LCFA always originates from the deep femoral artery, and the variations can originate from the common femoral artery, superficial femoral artery, lateral iliac artery, etc. The typical LCFA consists of ascending, transverse and descending branches, with a 35% probability of an oblique branch (10). Most of perforators originate from the d-LCFA. However, when originating from the oblique or transverse branch, the perforator vessels usually present as single with a higher location and hence a thicker flap, reducing the feasibility of a bi-paddle flap harvest despite the easier dissection. The location of the branches on the LCFA also varies. For example, regarding the descending branches, there are 17% descending branches that originate from the beginning of the LCFA, 6–13% from the deep femoral artery, and 1–6% from the common femoral artery (14). These variations affect the conversion method for bi-paddle flap harvest.
When harvest of a bi-paddle ALT flap according to preoperative design fails because the intraoperative findings vary from expected, we recommended that the conversion method should be based on the characteristics of the defects, the number of flap perforators and the vascular sources (Figure 5).
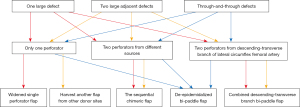
There are two conversion methods for a single-perforator flap to cover irregular, three-dimensionally large, or through-and-through defects (3,15). The de-epithelialized flap is supplied by the perforator by removing a piece of the epidermal flap (12,16). This is the preferred approach to covering adjacent or through-and-through defects. In Case 1, the bi-paddle flap was converted to a single-perforator de-epithelialized flap to cover the defects and eliminate lumen (7,17).
To cover only one large irregular defect, a bi-paddle flap with two subunits could reduce donor-site morbidity for primary closure. However, when variations of a single perforator are encountered, a widened-single-perforator flap can be elevated for only one large defect with skin grafting to the donor site, as in Case 3. The widened-single-perforator flap is easy to harvest by converting the two paddles into a single paddle, but this increases the need for skin grafting and morbidity to the donor site. Moreover, it is only suitable for a single large or irregular defect, not for multiple defects. The residual wound should be repaired by skin graft or by harvesting another flap from another donor site.
For variations of two perforators, if they originate from different vessels, the sequential chimeric flap is considered appropriate, as in Case 2. This approach allows for flexible design, a larger cutaneous area, and low donor site morbidity (7,17); primary closure can be achieved. However, hemodynamic resistance may occur in the distal flap after vascular anastomosis, thus increasing the possibility of vascular compromise. In fact, the selection of conversion method should take into account the characteristics of the defects. In the case of large or irregular defects, the sequential chimeric flap or widened-single-perforator flap are considered appropriate. For two adjacent or through-and-through defects, the sequential chimeric flap or de-epithelialized bi-paddle flap are suitable, and for two large adjacent defects, the sequential chimeric flap is the preferred one.
If both perforators originate from the LCFA, the combined transverse-and-descending branches flap can be harvested, as in Case 4. This flap is split into two subunits with the transverse and descending branches respectively. The LCFA is interlaced with femoral nerve branches, and is necessary to avoid disturbance of the femoral nerve; otherwise, the muscle force of the quadriceps can be lost. The donor site can undergo primary closure and has less morbidity.
Study limitations
This series is a retrospective analysis of only 9 cases with differences in the patients. Despite of this, we believe that our techniques are a reliable option to overcome the complexity of the vasculature of the ALT flap, increase flap survival and minimize complications, when failure and difficulties of bi-paddle ALT flap harvest present intraoperatively. More cases and multi-center studies are still needed to summarize and optimizing the flap transfer in order to overcome such failure intraoperatively.
Conclusions
The variation in the number and source of perforators of the LCFA mostly affects bi-paddle ALT flap harvest. To successfully harvest the flap when variations are encountered intraoperatively, redesign and modification will ensure intra-/postoperative success and reduce donor-site morbidity.
Acknowledgments
We sincerely appreciate the technical support and assistance of Bo Wang, the photographer for this research.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1118/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1118/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1118/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the 920th Hospital of Joint Logistics Support Force of Chinese PLA (the research registry No. E-R 2014-079-01) and informed consent was taken from all the patients or their family members.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wong CH, Wei FC. Anterolateral thigh flap. Head Neck 2010;32:529-40. [PubMed]
- Hsu CC, Loh CYY, Wei FC. The Anterolateral Thigh Perforator Flap: Its Expanding Role in Lower Extremity Reconstruction. Clin Plast Surg 2021;48:235-48. [Crossref] [PubMed]
- He J, Qing L, Wu P, et al. Individualized design of double skin paddle anterolateral thigh perforator flaps to repair complex soft tissue defects of the extremities: An anatomical study and retrospective cohort study. J Plast Reconstr Aesthet Surg 2021;74:530-9. [Crossref] [PubMed]
- Fukuta T, Ichikawa Y, Mizuno H, et al. Split-skin Paddle Anterolateral Thigh Flap for Reconstruction of Giant Dermatofibrosarcoma Protuberans in Groin. Plast Reconstr Surg Glob Open 2019;7:e2528. [Crossref] [PubMed]
- Ahmad QG, Yadav PS, Shankhdhar VK, et al. Anterolateral Thigh Twin Free Flaps from a Single Donor Site-a Modification Based on the Oblique Branch of the Lateral Circumflex Femoral Artery. Indian J Surg 2014;76:165-8. [Crossref] [PubMed]
- Shivanand NB, Chavre S, Chandrashekar NH, et al. Bi-paddled radial forearm free flap in the reconstruction of bilateral buccal mucosal defects-a case series. Oral Surg Oral Med Oral Pathol Oral Radiol 2016;122:e107-9. [Crossref] [PubMed]
- Tan NC, Shih HS, Chen CC, et al. Distal skin paddle as a monitor for buried anterolateral thigh flap in pharyngoesophageal reconstruction. Oral Oncol 2012;48:249-52. [Crossref] [PubMed]
- Marsh DJ, Chana JS. Reconstruction of very large defects: a novel application of the double skin paddle anterolateral thigh flap design provides for primary donor-site closure. J Plast Reconstr Aesthet Surg 2010;63:120-5. [Crossref] [PubMed]
- Okazaki M, Tanaka K, Kodaira S, et al. One-stage transfer of 2 paddles of thoracodorsal artery perforator flap with 1 pair of vascular anastomoses for Barraquer-Simons syndrome. J Craniofac Surg 2012;23:883-5. [Crossref] [PubMed]
- Deng C, Nie K, Wei Z, et al. Is the Oblique Branch a Preferable Vascular Pedicle for Anterolateral Thigh Free Flaps? J Reconstr Microsurg 2018;34:478-84. [Crossref] [PubMed]
- Wong CH. The oblique branch trap in the harvest of the anterolateral thigh myocutaneous flap. Microsurgery 2012;32:631-4. [Crossref] [PubMed]
- Kubo T, Osaki Y, Hattori R, et al. Reconstruction of through-and-through oromandibular defects by the double-skin paddle fibula osteocutaneous flap: can the skin paddle always be divided? J Plast Surg Hand Surg 2013;47:46-9. [Crossref] [PubMed]
- Lee YC, Chen WC, Chou TM, et al. Anatomical variability of the anterolateral thigh flap perforators: vascular anatomy and its clinical implications. Plast Reconstr Surg 2015;135:1097-107. [Crossref] [PubMed]
- Lakhiani C, Lee MR, Saint-Cyr M. Vascular anatomy of the anterolateral thigh flap: a systematic review. Plast Reconstr Surg 2012;130:1254-68. [Crossref] [PubMed]
- Song D, Li J, Pafitanis G, et al. Bilateral Anterolateral Thigh Myocutaneous Flaps for Giant Complex Chest Wall Reconstruction. Ann Plast Surg 2021;87:298-309. [Crossref] [PubMed]
- Shimbo K, Okuhara Y, Yokota K. Dual Purpose of De-Epithelialized Latissimus Dorsi Musculocutaneous Flap for Treatment of Chronic Frontal Sinusitis and Frontal Bone Defect. J Craniofac Surg 2021;32:1122-5. [Crossref] [PubMed]
- Ahmed A, Visavadia BG, Farrell R, et al. Use of double skin paddle for pharyngoesophageal reconstruction using tubed radial forearm free flap. Br J Oral Maxillofac Surg 2014;52:661-3. [Crossref] [PubMed]
(English Language Editor: K. Brown)


