
The efficacy and safety of chimeric antigen receptor T cells in digestive system cancers: a systematic review and meta-analysis
Introduction
Digestive system cancer (DSC) is a general term for a series of malignancies in digestive tracts and some related organs. The main types of DSC are esophageal cancer, gastric cancer, colorectal cancer, liver cancer, pancreatic cancer, gallbladder cancer. According to data from Global Cancer statistics (GLOBOCAN) 2018, colorectal cancer has the third highest incidence among all malignancies (10.2%) in both genders combined and the highest incidence rate among all DSCs, followed by gastric cancer (5.7%; 6th among all cancers), liver cancer (4.7%; 7th among all cancers), esophageal cancer (3.2%; 8th among all cancers), pancreatic cancer (2.5%; 13th among all cancers). Colorectal cancer also has the second highest mortality rate among all cancers (9.2%) and the highest mortality rate among all DSCs, followed by liver cancer (8.2%), stomach cancer (are 8.2%), esophageal cancer (5.3%; 6th among all cancers), pancreatic cancer (4.5%; 7th among all cancers) (1).
The most common treatments for DSC include surgery, chemotherapy, and immunotherapy (2). Most patients with DSC are diagnosed at a late stage due to the challenges associated with early diagnosis (3). Even with a successful operation and an effective treatment strategy, the general prognosis is poor. Hepatocellular carcinoma (HCC) has strong and broad resistance to cytotoxic chemotherapy, and the success of surgical intervention (liver resection, percutaneous ablation, trans-arterial chemoembolization) is highly dependent on the tumor size, tumor location, and the general condition of the patient (4). For advanced-stage cholangiocarcinoma, conventional systemic therapy (gemcitabine and cisplatin) is not effective, with a median overall survival (OS) of approximately 1 year (5). The therapeutic strategy for advanced-stage gastric cancer includes chemotherapy as a first- and second-line treatment. Although some specific targets, such as human epidermal growth factor receptor 2 (HER-2), have been identified, and trastuzumab combined with chemotherapy is used as a novel first-line treatment for advanced HER-2-positive stomach cancer, treatment strategies are still limited, and survival is still poor (5–20% 5-year survival and 10-month median OS) (6-8). For pancreatic cancer, over 50% of patients are diagnosed at an advanced stage, and systemic therapy (fluorouracil, folinic acid, irinotecan, oxaliplatin) only plays a role in supportive care to ameliorate symptoms afflicting patients (9-11). Pancreatic ductal adenocarcinoma has the poorest relative survival compared to other solid malignancies, and has been estimated to become the second leading cause of cancer-specific mortality in the United States by 2030 (9). Therefore, a novel pattern of systemic therapy for late-stage DSC is necessary.
Chimeric antigen receptor T cells (CAR-T cells) are state-of-the-art adoptive T cell immunotherapy. The CAR-T cells can precisely target tumor antigens independent of major histocompatibility molecule complexes. They are derived from T cells that are genetically engineered with a CAR-base sequence into the T cell genome. Unlike traditional T cells, CAR-T cells express a CAR receptor consisting of a single-chain variable fragment (scFv) that can directly bind to antigens on target tumor cells as well as transmembrane and intracellular signaling domains. To date, there are 4 generations of CAR-T cells. The first generation only has a CD3ζ domain. A costimulatory domain (CD28/4-1BB) and 2 costimulatory domains (CD28, 4-1BB, OX-40, ICOS) have been added to the second and third generations, respectively (12). Based on the third generation, the fourth generation is equipped with a cytokine-producing cassette. The T cells can produce and secrete cytokines as soon as they specifically target tumor cells. The CAR-T cell therapy has had great success in the treatment of hematological malignancies, especially in chemotherapy-resistant or refractory leukemia and lymphoma (13). For solid tumors, almost 200 clinical trials have been performed globally; however, the efficacy of the CAR-T regimen is not satisfactory (14). Phase I and II clinical trials of CAR-T cell therapy are underway for lung cancer and malignant brain tumors.
Various tumor antigens have been investigated for the treatment of lung cancers, including carcinoembryonic antigen (CEA), glypican-3 (GPC3), mucin 1 (MUC1), prostate stem cell antigen (PSCA) (15,16). Due to their mortality, brain tumors are also popular targets for CAR-T cell therapy. Glioblastoma multiforme and neuroblastoma are two major types of brain malignancies for which intensive preclinical and clinical research has been conducted. Unfortunately, no remarkable clinical response has been reported in clinical trials for brain tumors (17,18). Unlike lung and brain tumors, DSCs encompasses a large group of diseases. Poor prognosis during the era of conventional therapy for advanced DSC has prompted scientists to explore novel immunotherapies. Several clinical trials have been performed to evaluate the efficacy of different CAR-T cells in different types of DSCs. Compared to hematological malignancies, target antigens are more diversified in different types of solid tumors, thereby creating heterogeneous treatment patterns for CAR-T therapy, and various responses have occurred in different clinical studies (19). Moreover, tumor antigens expressed on solid tumors can also be produced by normal tissue. When targeting antigens in normal tissue, CAR-T cell therapy will cause side effects depending on which organs or tissues are involved, resulting in discrepancies in the types of adverse effects shown in different clinical studies that use CAR-T cells to treat solid tumors in a variety of organs and tissues (19,20).
Due to the wide range of elements included in different studies, it is necessary to perform a collective analysis of the impact of CAR-T cells on tumor treatment and the safety of patients. Although some studies have performed systematic reviews and meta-analyses of CAR-T cell treatment of solid tumors, few have summarized the integrated data (response rate and adverse effects) from DSC studies specifically (21,22). In this paper, we collected completed clinical trials of CAR-T cell therapy for various types of DSC and conducted a systematic review and meta-analysis of the clinical response and safety of this novel immunotherapy. We present the following article in accordance with the PRISMA reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-5019/rc).
Methods
Information sources and search strategy
We searched the PubMed, Cochrane, Embase, Web of Science databases from their inception to 1 October 2020. The following keywords were used to identify studies of interest: “chimeric antigen receptor T cell”, “CAR-T cell”, “cancer”, “neoplasm”, “malignancy”. The goal was to identify published clinical trials (randomization was not restricted) of the response and adverse events of CAR-T cells in the treatment of DSC. Only studies demonstrating drug response and adverse events were included. Only English language articles were included. The detailed search terms are provided in Table S1.
We selected studies based on the population, intervention, comparison, outcome, study design (PICOS) strategy. A study was included if it contained all of the following: (I) patients with DSC (nonhematological or nonlymphocytic); (II) interventions including only CAR-T cell therapy; (III) outcomes, such as clinical response [complete remission (CR), partial remission (PR), stable disease (SD), or progressive disease (PD)] and safety data of CAR-T cell therapy; and (IV) studies designed as clinical trials (retrospective or prospective studies). A study was excluded if any of following criteria were met: (I) patients with solid malignancies other than DSC, hematological malignancies, lymphocytic malignancies, or nonmalignant disorders; (II) a study of intervention other than CAR-T cell therapy; (III) duplicated data; (IV) not written in English; (V) a basic study, such as animal studies and pure bioinformatics studies; (VI) other types of literature (e.g., reviews, comments, chapters, systematic review/meta-analysis, correspondence, erratum, news, summary, protocols without original data); (VII) meeting abstract or case report; (VIII) absence of clinical outcome; and/or (IX) a published ongoing clinical trial (with fewer than 3 enrolled participants).
Study selection, quality assessment, and data extraction
The search results were independently reviewed by 2 researchers (ZJZ and XL) who identified eligible full-text articles. Quality evaluation of the included studies was conducted according to the Institute of Health Economics (IHE) risk of bias tool (23,24). The results of the quality assessment were demonstrated by a risk-of-bias plot using Review Manager 5.3 (RevMan; The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark, 2014). The following data of interest were then extracted from the included studies: first author, publication year, country, number of total evaluable patients, phase of clinical trial, age, type of cancer, time of follow-up, survival, vector for transduction, T cell origin (autologous or not), time of cell culture, T cell treatment, methods of transduction, transduction efficiency (%), CAR-T cell dose, treatment prior to CAR-T cell therapy, lymphodepletion, T cell persistence, co-stimulation signaling, generation of CAR-T cells, tumor antigen, clinical response. Disagreements were resolved by careful discussion between the 2 researchers. The primary outcome was the response rate to CAR-T cell treatment. The definition of tumor response was based on Response Evaluation Criteria in Solid Tumors (RECIST) v 1.1 with related data originating from computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography-computed tomography (PET-CT) imaging. Immune-related response evaluation criteria in solid tumors (irRECIST) criteria and World Health Organization (WHO) criteria were used in 1 study (25,26). Specifically, the overall response rate (ORR) was the percentage of patients who achieved CR and PR in the whole evaluable population. The clinical benefit rate (CBR) equaled the percentage of patients who achieved CR, PR or SD. The secondary outcome was the occurrence rate of various adverse events after CAR-T cell therapy in patients with DSC. The grading and nomenclature of adverse events were derived from Common Terminology Criteria for Adverse Events version 2.0/3.0/4.0 (CTCAE v. 2.0/3.0/4.0).
Statistical analysis
We used the “metaprop” function by installing the “meta” package to calculate single proportions (the response rate, including ORR and CBR, as well as the occurrence rate of adverse effects) in each study. The entire analysis procedure was completed using R version 4.0.2 (The R Foundation for Statistical Computing, Vienna, Austria). We performed a test of heterogeneity for the primary outcomes. The “double arcsin” transition of raw data was performed before conducting a pooling analysis of ORR and CBR. If the I2 statistic was less than 50% and the P-value was greater than 0.1, the proportions of these studies were recognized as homogenous, and the integrated proportion was calculated by a fixed effects model; if the I2 was greater than 50%, the random effects model was used. Forest plots were generated by the “forest” module. For heterogeneous results, subgroup analysis was conducted to clarify the potential relationship between each variable and the heterogeneity and possible factor(s) contributing to this heterogeneity. We also performed influence analysis using the “metainf” module to identify whether the results from certain studies affected the overall value. Funnel plots and Egger’s tests were used to report on the publication bias of each study. Except for the test of heterogeneity, all the other tests were 2-tailed, and P<0.05 was considered statistically significant.
Results
Study selection
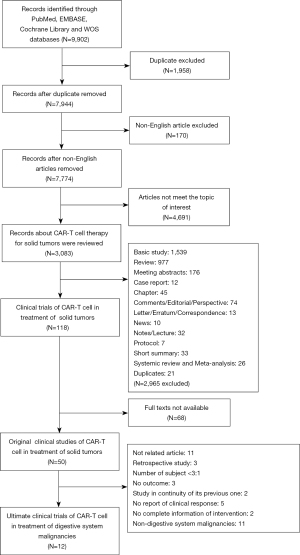
Publications were collected according to our search strategy. After excluding duplicates and non-English articles, we finally selected 7,774 studies for further analysis. Screening by title and abstract, we removed articles of nontumor diseases, hematological malignancies, lymphoma, and immunotherapy other than CAR-T cell treatment. Subsequently, 3,083 studies covering the area of CAR-T cell treatment for solid tumors were checked via reading of titles and abstracts. After filtering out reviews, basic studies, meeting abstracts, case reports, chapters, and other nonoriginal papers, we collected 118 original clinical studies of CAR-T cell therapy in the treatment of solid tumors. Among them, 68 full-text articles were not available. The remaining 50 full-text articles of clinical studies were checked. Ultimately, a total of 12 clinical trials (144 patients) of CAR-T cell therapy for DSC met the full eligibility criteria. The process of literature screening is shown in Figure 1. The quality assessment of these 12 studies is illustrated as bar plots and traffic-light plots in Figures S1,S2, respectively.
Study characteristics
The baseline characteristics of these 12 trials (144 patients) are summarized in Table 1 (25-36). All studies were phase I clinical trials that enrolled patients with DSC. The main intervention was first- or second-generation autologous CAR-T cells. No control group was set in these trials. These studies were published between 2015 and 2020, and the research teams were mainly from China and the United States. The sample size of the studies ranged from 3 to 23 participants. The median age of the patients in each study was between 50 and 69 years old. For the production of CAR-T cells, the sources of adoptive cells were all derived from patients via leukapheresis. Subsequent T cell expansion lasted for approximately 7 days (30) to 17 days (25) when bead-immobilized or non-bead anti-CD3/CD28 antibody or OKT-3 antibody was used to stimulate T cell growth and proliferation. A total of 7 trials added interleukin-2 (IL-2), while one study (35) added interferon-γ (IFN-γ) for increased T cell stimulation. Expanded T cells were then transduced either by lentivirus (8 studies) or by retrovirus (4 studies). The mean efficiency of transduction was up to 93.5% in the Beatty study, while it was also as low as 9.9% in the Feng study. For the final immunotherapy product, two studies used first-generation CAR-T cells, while the other 10 studies treated patients with second-generation CAR-T cells. Among studies using second-generation CAR-T cells, six transduced vectors containing a 4-1BB (CD137) co-stimulation domain, whereas the other four studies used vectors with a CD28 co-stimulation domain. Before CAR-T cell infusion, patient preparation was necessary to enhance CAR-T cell persistence and cytotoxicity. In 3 studies, patients were injected with IL-2 to strengthen CAR-T cell efficacy, and patients had received chemotherapy (cyclophosphamide + nab-paclitaxel/fludarabine) for lymphodepletion to ensure longer T cell persistence. Different studies had various durations of T cell persistence, and 11 studies had reported their outcomes. The CAR-T cells persisted less than one month in six studies and lasted more than one month in the other five studies. In six studies using 4-1BB CAR-T cells, three reported a persistence of CAR-T cells for more than one month. In four studies investigating CD28 CAR-T cells, two reported a more than one-month persistence of CAR-T cells.
Table 1
| Author, year | Country | N | Phase | Age, (y) median [range] | Cancer type | Vector | T cell origin | Cell culture (days) | T cell treatment | Transduction method | Transduction efficiency | CAR-T dose (cells/cells/m2 or cells/kg) | Previous treatment | IL-2 to patients | Lymphodepletion | T cell persistence | Co-stimulatory domain | CAR-T generation | Tumor antigen | Response |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beatty, G. L 2018 | US | 6 | I | 61 [50–71] | Chemotherapy-refractory metastatic, PDAC | CD3ζ | Auto | 8–12 | CD3/CD28 beads | Lentiviral | 93.5% (92.0–98.6%) | 1 to 3×108/m2 | Chemotherapy | No | No | 20–30 d | 4-1BB | 2 | MSLN | PD 4, SD 2 |
| Wang, Y 2018 | China | 23 | I | 56 [32–66] | Advanced metastatic: HCC, pancreatic carcinoma, CRC | CD3ζ, CD137, CD8 | Auto | <14 | Anti-CD3-mAb, IL-2 | Lentiviral | 11.23–56.47% | 0.05×106–2×106/kg | Surgical resection, radiotherapy, targeted therapy | No | Cyclophosphamide nab-paclitaxel | >2 months | 4-1BB | 2 | CD133 | 3 PR, 14 SD, 6 PD |
| Shi, D 2020 | China | 13 | I | 51 [34–70] | Relapsed HCC | CD3ζ, CD8, CD28 | Auto | 10–13 | Beads | Lentiviral | 64.4% (41.4–88.4%) | 1×105–2×109/kg | Surgical resection, radiotherapy, targeted therapy, chemotherapy, TCM, TACE/alcohol injection/microwave ablation | No | Cyclophosphamide fludarabine | >140 d | CD28 | 2 | GPC-3 | 2 PR, 2 SD, 5 PD, 4 NA |
| Thistlethwaite, F. C 2017 | UK | 14 | I | 46 [36–66] | Advanced and treatment-insensitive: esophagus adenocarcinoma, gastric adenocarcinoma, caecum adenocarcinoma, colon adenocarcinoma, rectal adenocarcinoma, pseudomyxoma peritonei, gastro-esophageal, junction, pancreas adenocarcinoma | CD3ζ | Auto | 7 | OKT3, IL-2 | Retroviral | 20.1–37.3% | 0.1×109–1.32×109 | Surgical resection, radiotherapy, targeted therapy, chemotherapy | Yes | Fludarabine cyclophosphamide | 14–30 d | No | 1 | CEA | 7 SD, 7 PD |
| Ko, A. H 2020 | US | 3 | I | 50 [38–64] | Advanced pancreatic adenocarcinoma | CD3ζ, CD137 | Auto | NA | CD3/CD28 beads | Lentiviral | 37% (MSLN) 22% (CD19) | 3×107/m2 | Radiotherapy, chemotherapy, targeted therapy | No | Cyclophosphamide | <28 d | 4-1BB | 2 | MSLNCD19 | 1 SD |
| Katz, S. C 2020 | US | 8 | I | 54.5 [39–65] | Colon adenocarcinoma LM, rectal adenocarcinoma LM, pancreatic adenocarcinoma LM | CD3ζ, CD28 | Auto | 12–16 | OKT3 IL-2 | Retroviral | 60.4% (40.0–67.8) | NA | Chemotherapy | Yes | NA | NA | CD28 | 2 | CEA | 1 SD, 6 PD |
| Zhang, C 2017 | China | 10 | I | 58 [48.8–67] | Metastatic colorectal cancers | CD3ζ, CD28 | Auto | 12–14 | Anti-CD3-mAb, anti-CD28-mAb, IL-2 | Lentivirus | 33.7% (14.7–43.2%) | 2.5×107–1.5×1010 /kg | Surgical resection, chemotherapy, radiotherapy | No | Cyclophosphamide, fludarabine | 4–6 w | CD28 | 2 | CEA | 7 SD, 2 PD, 1 NA |
| Katz, S. C 2015 | US | 6 | I | 54.5 [51–66] | Colon adenocarcinoma LM, ampullary adenocarcinoma LM | CD3ζ, CD8, CD28 | Auto | 10–14 | OKT-3, IL-2 | Retroviral | 53.2% (10.4–63.5%) | 1×108, 1×109, 1×1010 | Chemotherapy | Yes | No | <3 d | CD28 | 2 | CEA | 1 SD, 4 PD |
| Feng, K 2017 | China | 11 | I | 61 [50–75] | Advanced/relapsed/metastatic: biliary tract cancers, pancreatic cancers | CD3ζ, CD8, CD137 | Auto | 10 | Anti-CD3-mAb, IFN-γ, IL-2 | Lentiviral | 9.9% (5.5–11.4%) | 2.1×106/kg (1.4–3.8×106/kg). | Surgical resection, chemotherapy, radiotherapy, targeted therapy | No | Nab-paclitaxel. cyclophosphamide | >30 d | 4-1BB | 2 | HER-2 | 1 PR, 5 SD, 5 PD |
| Guo, Y 2018 | China | 19 | I | 57 [39–70] | Advanced, relapsed and metastatic: cholangiocarcinoma, gallbladder carcinoma | CD3ζ, CD8, CD137 | Auto | 10 | Anti-CD3-mAb, IL-2 | Lentivirus | 8.6% (mean, 6.3–11.2%) | 2.65×106/kg (0.8–4.1×106/kg) | Surgical resection, chemotherapy, radiotherapy | No | Nab-paclitaxel, cyclophosphamide | <1 m | 4-1BB | 2 | EGFR | 1 CR, 10 SD, 6 PD, 2 NA |
| Haas, A. R 2019 | US | 15 | I | 69 [48–75] | Persistent or recurrent: malignant pleural mesothelioma, ovarian carcinoma, pancreatic ductal adenocarcinoma. | CD3ζ, CD137 | Auto | 9–10 | CD3/CD28 beads | Lentivirus | 24.7% (mean, 15.5–35.7%) | (1–3)×107/m2, (1–3)×108/m2 | Chemotherapy, targeted therapy | No | Cyclophosphamide | 1–6 m | 4-1BB | 2 | MSLN | 11 SD |
| Hege, K. M 2017 | US | 16 | I | No | Metastatic colorectal cancers with LM | CD3ζ, CD4 | Auto | 17 | CD3/CD28 beads, IL-2 | Retroviral | 38% (mean) | 108–1010 | Surgical resection, chemotherapy, radiotherapy | No | No | ≤14 d | No | 1 | TAG-72 | 16 PD |
Auto, autologous; CAR-T, chimeric receptor T cell; CEA, carcinoembryonic antigen; CR, complete remission; CRC, colorectal carcinoma; EGFR, Epidermal Growth Factor Receptor; GPC-3, glypican-3; HCC, hepatocellular carcinoma; HER-2, human epidermal growth factor receptor 2; MSLN, mesothelin; NA, not available; PD, progressive disease; PDAC, pancreatic ductal adenocarcinoma; PR, partial remission; SD, stable disease; TAG-72, tumor-associated glycoprotein-72; UK, the United Kingdom; US, the United States.
Among these 12 studies, the main types of cancers included gastric carcinoma, colorectal carcinoma, HCC, pancreatic ductal adenocarcinoma, biliary tract carcinoma. Mesothelin, CD133, Glypican-3 (GPC-3), CEA, HER-2, epidermal growth factor receptor (EGFR), tumor-associated glycoprotein-72 (TAG-72) were cancer-specific antigens targeted by their corresponding CARs. The CEA was the most popular target in all these studies, and it was expressed in different types of cancer.
Response rate of CAR-T therapy
In our analysis, the response rate was calculated in two forms, namely, ORR and CBR. The ORR was the percentage of patients who achieved CR or PR in the whole evaluable population. The CBR equaled the number of patients with CR, PR, or SD in the total evaluable population. In 12 studies, one patient achieved CR (0.69%), six patients achieved PR (4.2%), and 61 patients (42.3%) had SD. The ORR was 2% [95% confidence interval (CI): 0% to 6%] according to the fixed effect model (I2=0%; P=0.72; Figure 2), whereas the CBR was 42% (95% CI: 24% to 61%) according to the random effects model (I2=77%; P<0.01; Figure 3).

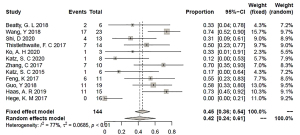
Due to heterogeneity in the pooled analysis of CBR, influence analysis using the leave-one-out method was conducted. The proportions were unaffected by the removal of any one of these studies, fluctuating between 38% and 50%, as illustrated in Figure 4. Based on this analysis, subgroup analysis was performed. The major baseline characteristics we selected to use in the subgroup analysis were co-stimulation (4-1BB and CD28), generation of CAR-T cells (second and first generation), IL-2 infusion for patients (no and yes), lymphodepletion (no/NA and yes), persistence of CAR-T cells (<1 vs. ≥1 month), transduction (lentiviral and retroviral), and IL-2 stimulation of T cells (no/NA and yes). The results of the subgroup analysis demonstrated that the sources of heterogeneity in our meta-analysis were co-stimulatory signal (P=0.0449), lymphodepletion (P=0.0002), duration of persistence (P=0.0443), and transduction method (P=0.0165) (Table 2). The forest plots are shown in Figures S3-S8. We observed that second generation CAR-T cells with the 4-1BB molecule achieved a better pooled CBR than those with the CD28 molecule (63.18% vs. 37.29%). Patients with lymphodepletion prior to CAR-T cell infusion responded better than those without lymphodepletion or related information (59.25% vs. 10.00%). The CAR-T cells with a longer persistence (≥1 month) were more beneficial for patients than CAR-T cell with a shorter persistence (61.81% vs. 29.77%). Regarding CAR-T cell production, transduction with lentivirus exerted a stronger effect than transduction with retrovirus (58.42% vs. 15.67%).
Table 2
| Subgroup | Studies | Pooled proportion | 95% CI | Heterogeneity (I2) | P |
|---|---|---|---|---|---|
| Co-stimulation | 0.0449 | ||||
| 4-1BB | 6 | 0.6318 | 0.5074–0.7492 | 3.7% | |
| CD28 | 4 | 0.3279 | 0.1013–0.5975 | 58.0% | |
| Generation | 0.3310 | ||||
| 2 | 10 | 0.4960 | 0.3437–0.6485 | 54.4% | |
| 1 | 2 | 0.1714 | 0.0000–0.8147 | 92.6% | |
| Infusion of IL-2 | 0.3157 | ||||
| No | 9 | 0.4631 | 0.2409–0.6921 | 81.0% | |
| Yes | 3 | 0.2836 | 0.0699–0.5525 | 43.5% | |
| Lymphodepletion | 0.0002 | ||||
| No/NA | 4 | 0.0965 | 0.0000–0.3137 | 50.9% | |
| Yes | 8 | 0.5925 | 0.4760–0.7047 | 21.6% | |
| Persistence | 0.0443 | ||||
| <1 month | 6 | 0.2977 | 0.0833–0.5596 | 68.1% | |
| ≥1 month | 5 | 0.6181 | 0.4501–0.7743 | 46.4% | |
| Transduction | 0.0165 | ||||
| Lentiviral | 8 | 0.5842 | 0.4538–0.7097 | 29.4% | |
| Retroviral | 4 | 0.1567 | 0.0000–0.4772 | 78.0% | |
| IL-2 stimulation | 0.7197 | ||||
| No/NA | 4 | 0.4626 | 0.2116–0.7220 | 48.2% | |
| Yes | 8 | 0.4001 | 0.1723–0.6497 | 83.3% | |
CI, confidence interval.
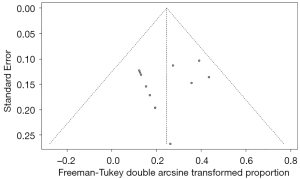
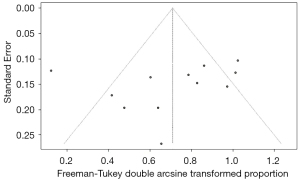
Regardless of the ORR or CBR analysis, no publication bias was found according to the funnel plot (Figures 5,6), which was validated by Egger’s test (P=0.4628 for the ORR test and P=0.3591 for the CBR test). The corresponding plots of Egger’s test for the analysis of ORR and CBR are shown in Figures S9,S10.
Adverse event analysis
As all studies were phase I clinical trials, thus, drug safety should not be neglected. Data on the number of patients experiencing adverse effects were extracted from all 12 studies except the Feng and Guo study (94 patients) (33,35). In total, 61 types of adverse events were summarized, and 21 side effects were selected for pooled analysis. According to Figure 7, five of the most frequent side effects were pyrexia (35%; 95% CI: 0.26 to 0.46), lymphopenia (30%; 95% CI: 0.21 to 0.40), pain other than abdominal pain (27%; 95% CI: 0.18 to 0.37), thrombocytopenia (27%; 95% CI: 0.18 to 0.37), and fatigue/malaise (23%; 95% CI: 0.15 to 0.33).
Discussion
With methodical screening of the literature and standardized statistical analysis, 12 studies with complete baseline clinical data and treatment response were collected. Our study generally included all types of DSC from the upper (esophageal and stomach) to lower digestive tracts (colon and rectum) and other important endocrinal and metabolic organs (liver, gallbladder, and pancreas). Although the language of the literature was restricted to English, the number of studies from Eastern and Western countries was almost equal (5 studies vs. 7 studies). According to the funnel plots and Egger’s tests, no publication bias was found in our study regardless of the pooled analysis of ORR or CBR, indicating that our data were comprehensive and our results were scientific and persuasive. The results showed that the ORR of CAR-T cell therapy for patients treated for DSC was 2% (95% CI: 0% to 6%). There was no heterogeneity in the analysis of CBR (I2=0%; P=0.72). This finding suggested that it was difficult for CAR-T cells to reduce or even extinguish carcinoma regardless of where the carcinoma originated or what kind of antigen was targeted by T cells. Despite a relatively low response rate by this method of calculation, we wanted to explore whether this novel treatment could be beneficial for patients. Adding the number of patients who achieved SD to the previous ORR resulted in the CBR value, and the pooled CBR was 42% (95% CI: 24% to 61%). Since nearly all the patients enrolled in these studies were those who had advanced, relapsed, or metastatic malignancies, the pooled treatment response of CAR-T cells was acceptable.
To date, two meta-analyses of CAR-T cell treatment in solid tumors have been published. In a meta-analysis of solid malignancies published by Hou in 2019, the ORR was 9% (95% CI: 4% to 16%), but significant heterogeneity was found (22). In another systematic review and meta-analysis, the pooled ORR of solid malignancies was 10% (95% CI: 5.1% to 18.9%), but no heterogeneity was found. Considering these two studies contained other solid malignancies more sensitive to CAR-T cells than DSC, our results validated that the ORR of patients with advanced, relapsed, or metastatic digestive cancer was less than 10%. However, the two meta-analyses lacked CBR results, which is an important element for evaluating the efficacy of immunotherapy, especially in patients with advanced, refractory, metastatic tumors.
Currently, immunotherapy faces great challenges in treating advanced/refractory or even metastatic digestive system malignancies. For instance, HCC is the fifth most common cancer and the fourth greatest cause of cancer-related death. For advanced HCC, traditional surgical treatments (local ablation, liver resection, transplantation) are not curative. Systemic therapy is more appropriate but not sufficiently diverse for advanced HCC treatment. First-line systemic therapy for HCC consists of the two tyrosine kinase inhibitors (TKIs), sorafenib and lenvatinib. As an oral immunotherapy, sorafenib has long been demonstrated by the phase III Sorafenib in Patients with Advanced Hepatocellular Carcinoma (SHARP) trial and Asian-Pacific (AP) trial to have a significant survival benefit compared to placebo (SHARP: median OS =10.7 months; P<0.001. AP trial: median OS =6.5 months; P<0.05). The response rate, however, was poor in both trials. No patient achieved CR in either trial, and the PR rates in the experimental group in the SHARP and AP trials were only 2% and 3%, respectively. The disease control rate of sorafenib (equal to CBR) was 43% and 35% (P<0.01) in the SHARP and AP trials, respectively (37,38).
Prolonged administration of sorafenib develops drug resistance within 6 months (38,39), and the detailed pathways and mechanisms of resistance remain unclear. Possible hypotheses include epithelial-mesenchymal transition of cancer cells, a hostile tumor microenvironment, activation of acquired tumor escape pathways such as the PI3K/Akt/mTOR pathway (40,41). Alternative treatment strategies are being explored.
Although it is not a first-line therapy, CAR-T cell therapy is a promising strategy for treatment of advanced solid malignancies. The CAR-T cells are new members of immunotherapy. There are similarities between TKIs and CAR-T cells, as both can specifically target cell surface molecules and exert inhibitory or cytotoxic functions. The CAR-T cells are superior to TKIs as they produce and secrete multiple cytotoxic cytokines. As a living drug, CAR-T cells also persist in tissue and circulation for a period of time, thereby resulting in a better drug efficacy and duration. Selection of tumor antigens of HCC is an important step for CAR-T cell therapy. GPC-3 is a proteoglycan that controls and regulates cell growth and division. As GPC-3-positive immunohistochemistry of liver tissue usually suggests a diagnosis of HCC, GPC-3 has been used as a tumor antigen for CAR-T cell therapy.
Similar to sorafenib, drug resistance can also occur in CAR-T cell therapy regardless of whether the cancers are hematological or solid. The main reasons include antigen escape, heterogeneous antigen patterns, an immunosuppressive microenvironment where Tregs, myeloid-derived suppressive cells, tumor-associated macrophages, immunosuppressive checkpoint receptors/ligands play a key role (12). In our study, the ORR and CBR of CAR-T cell therapy for DSC were 2% and 42%, respectively, which were similar to the results of the SHARP trial and the AP trial. In 2020, Shi studied patients with relapsed HCC who were treated with anti-GPC-3 CAR-T cells, and reported that two patients achieved PR and 2 patients achieved SD with an ORR of 15.4% and CBR rate of 30.8%. The response rate of CAR-T cells for HCC was greater than that of TKIs, and it should be noted that the sample size of this CAR-T cell clinical trial was much smaller than that of the SHARP and AP trials. Moreover, the condition of patients was different because patients enrolled in the SHARP and AP trials had late-stage HCC, while the Shi study recruited patients with relapsed HCC. Thus, CAR-T cell therapy is needed to improve the treatment of advanced, relapsed, and even metastatic solid carcinoma.
Through subgroup analysis of potential factors causing heterogeneity in the pooled calculation of CBR, we found 5 major potential elements, which were also factors affecting the efficacy of CAR-T cell therapy. First, we found that CAR containing a costimulatory domain of 4-1BB was more effective than CAR containing a costimulatory domain of CD28, which was partly supported by the findings of other studies. Long et al. (42) found that CD19 CAR T cells with 4-1BB signaling have a longer persistence than those with CD28 signaling. CAR-T cells with a 4-1BB domain reduce the expression of exhaustion molecules, and they secrete high amounts of cytokines and exert their cytotoxicity on malignant cells. The conclusion of this work was in line with other studies on CD19 or CD20 CAR-T cells for lymphoma treatment, revealing that the signaling pathway of the effect of 4-1BB is related to noncanonical nuclear factor-κB (NF-κB) signaling (43,44). Ying et al. (43) also found that CAR-T cells with 4-1BB are more tolerable than those with CD28. Similar results were found in the treatment of GPC-3-positive solid tumors, including HCC and hepatoblastoma, in which CAR-T cells with the 4-1BB costimulatory domain have much greater proliferation and cytotoxicity than those with the CD28 domain. However, CD28-equipped CAR-T cells are less vulnerable to immunosuppression of Treg cells (45). As a result, third generation CAR-T cells were produced by adding both CD28 and 4-1BB costimulatory domains.
Moreover, well-prepared lymphodepletion prior to CAR-T cell infusion is helpful for better CAR-T cell persistence and antitumor response. In the treatment of hematological malignancies, such as CD19+ B cell leukemia or lymphoma, lymphodepletion chemotherapy before immunotherapy was found to elongate T cell persistence and enhance their cytotoxic effect (46), with CRs more than 90% in B-ALL, more than 80% in non-Hodgkin lymphoma, and more than 70% in chronic lymphocytic leukemia. Researchers have found that artificial leukopenia by chemotherapy offers the following advantages: a spatial environment for exogenous T cells for growth and proliferation; inhibition of competitive usage of cytokines (IL-7 and IL-15), which are beneficial for T cell activity and survival, by other resident lymphoid stromal cells; and destruction of some common immunosuppressive cells, including Treg cells (37,47,48). These findings supported results from our study that lymphodepletion is beneficial for patients receiving CAR-T cell therapy.
Different media for transduction can also impact the treatment response. In our analysis, T cells transduced by lentivirus achieved a better response rate than those transduced by retrovirus. Currently, adoptive T cell transduction is mostly achieved using viral vectors due to the more complex molecular structure of the virus, precise gene integration, and stable transgene expression by viral vector transduction (49). The two main methods of viral transduction are γ-retrovirus and lentiviral vectors. Regarding safety issues, several preclinical studies have demonstrated that lentiviruses cause less genotoxicity than retroviruses (50,51). The reason is that integration sites of γ-retrovirus are preferential in proximity to active promoters in transcribed genes of any types of host cells. In contrast, integration sites of lentiviral vectors are more randomized throughout the whole transcriptional unit. Hence, γ-retroviral transduction has a higher risk of insertional mutagenesis than lentiviral transduction. On account of a safer integration site profile, lentiviral vectors are more common than γ-retrovirus in vector transduction. Theoretically, both γ-retrovirus and lentivirus are members of the Retroviridae family. The gag (expressing capsid proteins), pol (expressing reverse transcriptase and integrase), and env (producing envelope proteins) genes exist in the genome of all retroviruses. In contrast to other retroviruses, however, lentiviruses also possess two regulatory genes, namely tat and rev (52,53). Superior to other members of the Retroviridae family, lentiviruses also effectively infect quiescent cells and access the genome of host cells by transporting across the nuclear pore. Therefore, lentiviral transduction is more efficacious and popular than transduction of the γ-retrovirus in gene therapy (54-56).
In addition to treatment response, safety is another vital issue of a novel treatment. The main side effects of CAR-T cells in hematological malignancies or solid tumors include cytokine release syndrome (CRS), hemophagocytic lymphohistiocytosis (HLH)/macrophage activation syndrome (MAS), neurotoxicity (57,58). The inflammatory syndrome known as CRS is caused by acute and severe increases in multiple cytokines in circulation, including C-reactive protein (CRP), ferritin, IFN-γ, IL-1, IL-2, soluble IL2Rα, IL-4, IL-6, IL-8, IL-10, tumor necrosis factor-α (TNF-α), granulocyte/macrophage colony stimulating factor (GM-CSF) (13,59-61). The clinical presentations of CRS are pyrexia, malaise, fatigue, rash, hypotension, or lung edema (57). There are four grades of CRS, with grade 1 being the slightest grade and grade 4 the most severe (61,62). The diagnostic criteria of HLH/MAS are rapidly rising ferritin (>5,000 ng/mL), cytopenia, grade ≥3 elevation in aspartate transaminase/alanine aminotransferase (AST/ALT), bilirubin, grade ≥3 creatinine elevation, grade ≥3 pulmonary edema, and presence of hemophagocytosis in bone marrow or other organs (63). Neurotoxicity is associated with immune effector cells. The most common symptom is encephalopathy, including mild cognitive dysfunction, impaired attention, somnolence, and confusion. Other clinical features are tremor, apraxia, dysgraphia, focal weakness/numbness, seizures (clinical or subclinical), and diffuse cerebral edema. Of these manifestations, previously published studies have reported that encephalopathy is the most commonly encountered symptom (57,62).
In our meta-analysis, more than 60 adverse events during or after CAR-T cell therapy were reported in 10 studies (94 patients in total). Due to the small numbers of each type of side effect, the information of the other 2 studies (Feng and Guo study) (33,35) was not included in our pooled evaluation. We then selected 21 common adverse events and compared their frequencies. The five most common side effects were pyrexia (35%; 95% CI: 0.26 to 0.46), lymphopenia (30%; 95% CI: 0.21 to 0.40), pain other than abdominal pain (27%; 95% CI: 0.18 to 0.37), thrombocytopenia (27%; 95% CI: 0.18 to 0.37), and fatigue (23%; 95% CI: 0.15 to 0.33). The rate of abdominal pain was 18%, which was the eighth most common side effect (95% CI: 0.11 to 0.27). Other pain included myalgia and arthralgia. Pyrexia, pain, and fatigue/malaise were associated with CRS, while lymphopenia and thrombocytopenia were major components of HLH/MAS. Due to the large heterogeneity of adverse events in these 10 studies, and because some studies did not grade these side effects, we did not summarize these events in different grades.
Our study had several limitations. First, some of the studies enrolled patients with different types of cancers; as a result, we could not analyze the relationship between cancer types and response rates. Second, there was not sufficient information about the duration of follow-up and survival data for prognosis analysis. Third, although we summarized the dose of CAR-T cell infusion in each study in the form of a range, the units of each study were not unified, which made it difficult to perform a proper pooled analysis and subgroup analysis. Therefore, we failed to identify a safe and effective dose of CAR-T cells to obtain a better response of advanced and metastatic DSCs. Lastly, more clinical trials are necessary to evaluate the efficacy and safety of CAR-T cell therapy.
Conclusions
Our study suggested a poor ORR of CAR-T cell therapy in the treatment of DSC. Fortunately, nearly 50% of patients benefited from this drug. To increase efficacy and safety, more potential tumor antigens should be explored to avoid drug resistance and reduce the recurrence rate. The present study also demonstrated that 4-1BB co-stimulation, lymphodepletion, and lentiviral vector transduction increased the benefit rate of this drug. This finding can be helpful for subsequent research and development of CAR-T cells. The safety and adverse effects of CAR-T cells in our study of DSC were similar to those of studies where CAR-T cells had been used to treat blood and solid tumors. Close monitoring before and after infusion of CAR-T cells is essential. Above all, a more sophisticated CAR design, more useful tumor antigens, and a consistent treatment strategy are indispensable for future clinical trials to achieve significant progress.
Acknowledgments
We thank Prof. Changtai Zhu from Shanghai Jiao Tong University for helping and advising of data integration and analysis. We also thank Dr. C. Mullens and J. Gray from AME editing service for English Language polishing.
Funding: This work was supported by International Science and Technology Cooperation Projects (2016YFE0107100 and 2015DFA30650), CAMS Clinical and Translational Medicine Research Funds (2019XK320006), CAMS Innovation Fund for Medical Science (CIFMS) (2017-I2M-4-003 and 2018-I2M-3-001), Capital Special Research Project for Health Development (2014-2-4012), Beijing Natural Science Foundation (L172055 and 7192158), the Fundamental Research Funds for the Central Universities (3332018032) and National Ten-thousand Talent Program.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-5019/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-5019/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Song Y, Ye M, Zhou J, et al. Targeting E-cadherin expression with small molecules for digestive cancer treatment. Am J Transl Res 2019;11:3932-44. [PubMed]
- Fang X, Wang D, Pu K, et al. Diagnostic value of circulating lncRNAs as biomarkers of digestive system cancers: A systematic review and meta-analysis. Expert Rev Mol Diagn 2020;20:1051-62. [Crossref] [PubMed]
- Sangro B, Sarobe P, Hervás-Stubbs S, et al. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18:525-43. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Apicella M, Corso S, Giordano S. Targeted therapies for gastric cancer: failures and hopes from clinical trials. Oncotarget 2017;8:57654-69. [Crossref] [PubMed]
- Association JGC. Japanese Gastric Cancer Treatment Guidelines 2018 (ver. 5). 2018.
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Weledji EP, Enoworock G, Mokake M, et al. How Grim is Pancreatic Cancer? Oncol Rev 2016;10:294. [PubMed]
- Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol 2013;31:23-9. [Crossref] [PubMed]
- Zhao Z, Xiao X, Saw PE, et al. Chimeric antigen receptor T cells in solid tumors: a war against the tumor microenvironment. Sci China Life Sci 2020;63:180-205. [Crossref] [PubMed]
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Liu G, Rui W, Zhao X, et al. Enhancing CAR-T cell efficacy in solid tumors by targeting the tumor microenvironment. Cell Mol Immunol 2021;18:1085-95. [Crossref] [PubMed]
- Qu J, Mei Q, Chen L, et al. Chimeric antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer (NSCLC): current status and future perspectives. Cancer Immunol Immunother 2021;70:619-31. [Crossref] [PubMed]
- Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2014;2:112-20. [Crossref] [PubMed]
- Land CA, Musich PR, Haydar D, et al. Chimeric antigen receptor T-cell therapy in glioblastoma: charging the T cells to fight. J Transl Med 2020;18:428. [Crossref] [PubMed]
- Chung H, Jung H, Noh JY. Emerging Approaches for Solid Tumor Treatment Using CAR-T Cell Therapy. Int J Mol Sci 2021;22:12126. [Crossref] [PubMed]
- Nardo M, Motta TC, Colli LM, et al. Associação Brasileira de Hematologia, Hematologia, Hemoterapia e Terapia Celular Consensus on genetically modified cells. Review article: Cell therapy in solid tumors. Hematol Transfus Cell Ther 2021;43:S78-83. [Crossref] [PubMed]
- Martinez M, Moon EK. CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Front Immunol 2019;10:128. [Crossref] [PubMed]
- Grigor EJM, Fergusson D, Kekre N, et al. Risks and Benefits of Chimeric Antigen Receptor T-Cell (CAR-T) Therapy in Cancer: A Systematic Review and Meta-Analysis. Transfus Med Rev 2019;33:98-110. [Crossref] [PubMed]
- Hou B, Tang Y, Li W, et al. Efficiency of CAR-T Therapy for Treatment of Solid Tumor in Clinical Trials: A Meta-Analysis. Dis Markers 2019;2019:3425291. [Crossref] [PubMed]
- Guo B, Moga C, Harstall C, et al. A principal component analysis is conducted for a case series quality appraisal checklist. J Clin Epidemiol 2016;69:199-207.e2. [Crossref] [PubMed]
- Shuster JJ. Review: Cochrane handbook for systematic reviews for interventions. 2011;2:126-130.
- Hege KM, Bergsland EK, Fisher GA, et al. Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J Immunother Cancer 2017;5:22. [Crossref] [PubMed]
- Zhang C, Wang Z, Yang Z, et al. Phase I Escalating-Dose Trial of CAR-T Therapy Targeting CEA+ Metastatic Colorectal Cancers. Mol Ther 2017;25:1248-58. [Crossref] [PubMed]
- Beatty GL, O'Hara MH, Lacey SF, et al. Activity of Mesothelin-Specific Chimeric Antigen Receptor T Cells Against Pancreatic Carcinoma Metastases in a Phase 1 Trial. Gastroenterology 2018;155:29-32. [Crossref] [PubMed]
- Wang Y, Chen M, Wu Z, et al. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology 2018;7:e1440169. [Crossref] [PubMed]
- Shi D, Shi Y, Kaseb AO, et al. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials. Clin Cancer Res 2020;26:3979-89. [Crossref] [PubMed]
- Thistlethwaite FC, Gilham DE, Guest RD, et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol Immunother 2017;66:1425-36. [Crossref] [PubMed]
- Ko AH, Jordan AC, Tooker E, et al. Dual Targeting of Mesothelin and CD19 with Chimeric Antigen Receptor-Modified T Cells in Patients with Metastatic Pancreatic Cancer. Mol Ther 2020;28:2367-78. [Crossref] [PubMed]
- Katz SC, Hardaway J, Prince E, et al. HITM-SIR: phase Ib trial of intraarterial chimeric antigen receptor T-cell therapy and selective internal radiation therapy for CEA+ liver metastases. Cancer Gene Ther 2020;27:341-55. [Crossref] [PubMed]
- Guo Y, Feng K, Liu Y, et al. Phase I Study of Chimeric Antigen Receptor-Modified T Cells in Patients with EGFR-Positive Advanced Biliary Tract Cancers. Clin Cancer Res 2018;24:1277-86. [Crossref] [PubMed]
- Katz SC, Burga RA, McCormack E, et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clin Cancer Res 2015;21:3149-59. [Crossref] [PubMed]
- Feng K, Liu Y, Guo Y, et al. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell 2018;9:838-47. [Crossref] [PubMed]
- Haas AR, Tanyi JL, O'Hara MH, et al. Phase I Study of Lentiviral-Transduced Chimeric Antigen Receptor-Modified T Cells Recognizing Mesothelin in Advanced Solid Cancers. Mol Ther 2019;27:1919-29. [Crossref] [PubMed]
- Cui Y, Zhang H, Meadors J, et al. Harnessing the physiology of lymphopenia to support adoptive immunotherapy in lymphoreplete hosts. Blood 2009;114:3831-40. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Chen J, Jin R, Zhao J, et al. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Lett 2015;367:1-11. [Crossref] [PubMed]
- Chen KF, Chen HL, Tai WT, et al. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther 2011;337:155-61. [Crossref] [PubMed]
- Huang XY, Ke AW, Shi GM, et al. αB-crystallin complexes with 14-3-3ζ to induce epithelial-mesenchymal transition and resistance to sorafenib in hepatocellular carcinoma. Hepatology 2013;57:2235-47. [Crossref] [PubMed]
- Long AH, Haso WM, Shern JF, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 2015;21:581-90. [Crossref] [PubMed]
- Ying Z, He T, Wang X, et al. Parallel Comparison of 4-1BB or CD28 Co-stimulated CD19-Targeted CAR-T Cells for B Cell Non-Hodgkin's Lymphoma. Mol Ther Oncolytics 2019;15:60-8. [Crossref] [PubMed]
- Philipson BI, O'Connor RS, May MJ, et al. 4-1BB costimulation promotes CAR T cell survival through noncanonical NF-κB signaling. Sci Signal 2020;13:aay8248. [Crossref] [PubMed]
- Kegler A, Koristka S, Bergmann R, et al. T cells engrafted with a UniCAR 28/z outperform UniCAR BB/z-transduced T cells in the face of regulatory T cell-mediated immunosuppression. Oncoimmunology 2019;8:e1621676. [Crossref] [PubMed]
- Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017;130:2295-306. [Crossref] [PubMed]
- Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 2005;202:907-12. [Crossref] [PubMed]
- Ramos CA, Dotti G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin Biol Ther 2011;11:855-73. [Crossref] [PubMed]
- Qin DY, Huang Y, Li D, et al. Paralleled comparison of vectors for the generation of CAR-T cells. Anticancer Drugs 2016;27:711-22. [Crossref] [PubMed]
- De Palma M, Montini E, Santoni de Sio FR, et al. Promoter trapping reveals significant differences in integration site selection between MLV and HIV vectors in primary hematopoietic cells. Blood 2005;105:2307-15. [Crossref] [PubMed]
- Montini E, Cesana D, Schmidt M, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol 2006;24:687-96. [Crossref] [PubMed]
- Poorebrahim M, Sadeghi S, Fakhr E, et al. Production of CAR T-cells by GMP-grade lentiviral vectors: latest advances and future prospects. Crit Rev Clin Lab Sci 2019;56:393-419. [Crossref] [PubMed]
- Escors D, Breckpot K. Lentiviral vectors in gene therapy: their current status and future potential. Arch Immunol Ther Exp (Warsz) 2010;58:107-19. [Crossref] [PubMed]
- Bukrinsky MI, Sharova N, Dempsey MP, et al. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci U S A 1992;89:6580-4. [Crossref] [PubMed]
- Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J 1992;11:3053-8. [Crossref] [PubMed]
- Gallay P, Hope T, Chin D, et al. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci U S A 1997;94:9825-30. [Crossref] [PubMed]
- Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin 2020;70:86-104. [Crossref] [PubMed]
- Zhao Z, Chen Y, Francisco NM, et al. The application of CAR-T cell therapy in hematological malignancies: advantages and challenges. Acta Pharm Sin B 2018;8:539-51. [Crossref] [PubMed]
- Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016;126:2123-38. [Crossref] [PubMed]
- Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev 2019;34:45-55. [Crossref] [PubMed]
- Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 2018;15:47-62. [Crossref] [PubMed]
- Rivera AM, May S, Lei M, et al. CAR T-Cell-Associated Neurotoxicity: Current Management and Emerging Treatment Strategies. Crit Care Nurs Q 2020;43:191-204. [Crossref] [PubMed]
- Sadaat M, Jang S. Hemophagocytic lymphohistiocytosis with immunotherapy: brief review and case report. J Immunother Cancer 2018;6:49. [Crossref] [PubMed]




