Comparative study of tumor markers in patients with colorectal carcinoma before and after chemotherapy
Introduction
The US Centers for Disease Prevention and Control (CDC) identifies colorectal carcinoma (CRC) as the second leading cause of cancer-related deaths, among both sexes combined (1). Worldwide, over a million cases are reported each year. Population-based longitudinal studies show a rising trend in the incidence of CRC in India; it being the fourth most common cancer in males (7.7% incidence) and third most common in females (5.1% incidence), as per Globocan 2012 (2). An extensive literature review, done by European Group on Tumor Markers (EGTM-2007), concluded that for clinical use no biomarkers were recommended in CRC patients to improve treatment outcome (3).
Carcinoembryonic antigen (CEA) is a 180 kDa oncofetal glycoprotein, normally present in large intestine. Its level increases in most gastrointestinal malignancies, lung, breast and thyroid cancers (4). CEA is a glycosylphosphatidyl inositol (GPI)-cell surface anchored glycoprotein whose specialized sialofucosylated glycoforms serve as functional colon carcinoma L-selectin and E-selectin ligands, which may be critical to the metastatic dissemination of colon carcinoma cells (5). There is increasing evidence that prolactin (PRL) plays a role in several types of cancer in reproductive and non-reproductive tissues due to local production. The expression of both PRL and its receptor in human cancer cell lines of diverse origin indicates its action as an autocrine or paracrine growth factor (6). Otte et al. observed that there was an extensive PRL receptor expression in CRC tumor cells and it was significantly associated with tumor size, grade and histological subtype (7,8).
Colorectal carcinoma cells can also produce alfa feto protein (AFP), with raised levels in CRC due to blood-borne metastases from the tumor cells. All reported cases of CRC with raised AFP have very poor prognosis due to extensive liver and or lung metastases at the time of diagnosis (9). Human chorionic gonadotropin (hCG) was believed to be secreted only by the embryo. However, recent studies indicate that hCG is also secreted by CRC cells (10), and beta-hCG levels were found to be associated with lymph node metastasis in CRC (11), although it did not improve diagnostic accuracy in detecting recurrent CRC (12). Cancer antigen-125 (CA-125) was initially believed to be a specific biomarker for ovarian cancer, but recently it has been found in sera of patients with gastric, colorectal, and pancreatic adenocarcinomas. The level of CA-125 is raised in greater proportion of CRC cases (13). Serum testosterone level, on the other hand, may have an inverse association with the risk of CRC in men (14). Ferritin, prostate specific antigen (PSA) and free PSA have been included with other markers of CRC as diagnostic tools in some studies (15).
CEA is an already established diagnostic marker in CRC patients (16). However, there is an element of inconsistency observed across different studies evaluating the different diagnostic markers of CRC.
The objective of the current study was to compare the levels of serum CEA, PRL, AFP, total hCG, CA-125, testosterone, PSA and ferritin in diagnosed pre-treatment male cases of CRC with age-matched healthy male controls. We also aimed to compare select tumor markers in a group of CRC patients, before and after chemotherapy.
Materials and methods
The current hospital-based follow-up study was conducted in the Departments of Biochemistry and Radiotherapy in a tertiary care hospital in north India. Ethical clearance was obtained from the Institutional Ethical Committee. Informed written consents were obtained from all participants before commencement of the study.
Fifty one (n=51) male patients with colorectal carcinoma who attended the Radiotherapy Department between May, 2012 and June, 2013 for treatment (before chemotherapy) were enrolled in our study. All such cases were clinically diagnosed and further confirmed by histopathology. Fifty (n=50) age-matched healthy males, from among people accompanying the patients, were included as the comparison group. Four months of follow up was done for each case, during which patients received six cycles of chemotherapy with 5-flurouracil (5-FU) and oxaliplatin. No patient received radiotherapy. Four out of 51 patients were lost during this follow up. In the post-chemotherapy group (n=47), CEA, PRL, AFP, CA-125 and testosterone were measured and compared with the values obtained in the pre-chemotherapy stage for these patients.
Patients with galactorrhea, irritable bowel disease (IBD) (ulcerative colitis and Crohn’s disease), hypopituitarism, hypothyroidism, benign prostatic hypertrophy, prostate cancer, testicular cancer and liver disease were excluded from the study. Patients who were on methyldopa, metoclopramide, antidepressant, verapamil, reserpin, phenothiazine, resperidone and on any hormonal pills or iron tablets were also excluded.
Venous blood was collected from all cases (n=51) and controls (n=50) at the start of the study (pre-treatment stage). A total of 6 mL of venous blood was collected aseptically from the antecubital vein; three times (2 mL each time) with an interval of 15 minutes in the in appropriate vacutainer. Serum was separated by centrifugation at 3,000 rpm for 10 minutes. Serum from the three samples was later pooled by adding equal volume from each samples (17). These pooled serum samples were kept frozen at −20 °C until day of the assay and finally analyzed for tests. After four months, blood sample was again obtained from the cases who completed the chemotherapy, as described above (n=47).
Serum CEA, PRL, AFP, testosterone, CA-125, PSA, total hCG and ferritin levels were measured by direct chemiluminescence technique by using ADVIA Centaur assay, a competitive immunoassay (kit supplied by SIEMEN) [Normal range of CEA =0–2.5 ng/mL, PRL =2.1–17.7 ng/mL (for males), AFP =0–8 ng/mL, total hCG ≤10 mIU/mL, CA-125 =0–35 U/L, testosterone for males =241–827 ng/dL, PSA =0–4 ng/mL, and ferritin =22–322 ng/mL].
Surgical staging of CRC is based on the TNM classification determined by the spread of tumor (T), lymph nodes involvement (N) and, distant metastasis (M). Superficial lesions that do not involve regional lymph nodes and do not penetrate through the submucosa (T1) or the muscularis (T2) are designated as stage I (T1–2N0M0) disease; tumors that penetrate through the muscularis but have not spread to lymph nodes are stage II disease (T3N0M0); regional lymph node involvement defines stage III (TXN1M0) disease; and metastatic spread to sites such as liver, lung, or bone indicates stage IV (TXNXM1) disease (18).
Number of patients in each stage (TNM) were n=10 (stage I), n=17 (stage II), n=13 (stage III), and n=11 (stage IV). Levels of CEA, PRL, AFP, CA-125 and testosterone were analyzed according to four stages of the disease both in pre and post chemotherapeutic stages.
Sample size calculations
The sample size was calculated based on the CEA levels in patients and controls from a previous (unpublished) study at the Department of Biochemistry. A sample size of 47 in each arm had 90% power and 95% level of confidence to detect a mean CEA difference of 2.47 ng/mL between the CRC cases and healthy controls. Sample size was calculated using the n-Master v1.0 software developed by the Department of Biostatistics, Christian Medical College, Vellore, India.
Statistical analysis
The data were analyzed and represented as mean ± SD; students’ t test, paired t test and analysis of variance (ANOVA) were applied as tests of significance (2-tailed); P<0.05 was considered significant. In cases where ANOVA was significant, the post-hoc Bonferroni test was done. All analyses were performed using SPSS version 17.0 (SPSS, Inc., Chicago, Illinois).
Results
The mean age for the CRC case-group was 49.31 years, while it was 53.04 years in the control arm. Table 1 demonstrates the comparison of select tumor markers between CRC cases and controls. CEA (5.94±8.27 in cases vs. 2.5±0.79 in controls; P<0.05), PRL (28.12±13.39 in cases vs. 14.24±13.13 in controls; P<0.0001) and AFP (10.9±6.65 in cases vs. 4.02±1.26 in controls; P<0.0001) were significantly raised among cases in comparison to controls. On the contrary, testosterone level (237.63±46.19 in cases vs. 264.13±54.37 in controls; P<0.05) was significantly lower in cases. CA-125, though was raised in cases (37.91±22.18) compared to controls (31.69±8.86), it was not statistically significant. No significant differences were obtained in the other parameters (total hCG, PSA and ferritin levels).

Full table
Table 2 demonstrates the comparison between pre chemotherapy and post chemotherapy patient groups. Four-month treatment with chemotherapy significantly improved the biochemical parameters: CEA (P<0.05), AFP (P<0.0001) and CA-125 (P<0.05) levels substantially decreased with chemotherapy.
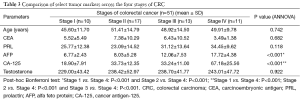
Additionally, we also tried to compare the levels of these five tumor markers across the four TNM stages of CRC (Table 3); AFP and CEA were raised significantly in stage IV in comparison to the other stages. However, as a note of caution, it should be understood that our study was not powered to study this sub-group analysis, and hence these results are only for academic discussion purposes.

Full table
Full table
Discussion
Tumor markers play an important role in evaluation as well as treatment of patients with cancer (19). Therefore in the assessment of the cancer prognosis and therapeutic response, these markers have a crucial role (20).
Carcinoembryonic antigen (CEA)
Persistently elevated levels of CEA, that are 5–10 times of upper reference limit strongly suggest the presence of colon cancer. CEA levels also correlate with the disease stage. During remission, CEA levels are stable. Raised CEA level after initial therapy is indicative of recurrences. The lead time for CEA elevation in clinical practice is five months (17). Patients with lymphovascular invasion and high preoperative CEA levels are likely to have a more aggressive clinical course (18). In our study, we found a statistically significant difference in CEA between the cases (raised) and controls; and also between the pre- (higher) and post-chemotherapy stages in the patients. This finding is consistent with established literature. However, the mean value of CEA in our patient population was strikingly low than what other studies have reported (4). This might because CEA is a nonspecific marker of CRC which may vary with geographical distribution and population genetic makeup.
Prolactin (PRL)
It has been stated that PRL may be the most significant independent prognostic factor influencing overall survival in CRC patients. Preoperative PRL levels showed excellent and significant correlation with response to therapy and progression of disease (19). Many researchers stated that PRL was an active participant in tumorogenesis (6). According to Otte et al., there might be some mitogenic effects of PRL which suggest a role for this hormone in a subgroup of CRCs. This action may be mediated by paracrine/autocrine pathways (8). Barrera had stated that PRL had a pleiotropic hormone action associated with the progression of various cancers, including CRC (21). Ilan et al. demonstrated PRL in human colon cancer and reported that all plasma PRL levels returned to normal after tumor resection, and remained so during a three-month follow-up (22). According to Neradugomma et al., higher levels of PRL-R (receptor) expression were observed in the colon cancers and cell lines compared to normal colonic epithelial cells. Cytokine signaling induced by PRL might be active in CRC, providing a new target for therapeutic targeting (23).
In our study, we found significantly increased levels of PRL in cases compared to controls; but such a finding was not observed in the pre- and post-chemotherapy contrast.
Several studies exhibit absence of any relationship between increased PRL levels and CRC. Wood et al. reported that serum PRL concentrations may occasionally be raised in CRC patients, but the tumor is not the source of hormone production (24). According to Carlson et al. serum PRL is not a useful marker for colon carcinoma, at least in patients in the United States (25). Indinnimeo stated that circulating PRL levels could not be taken as prognostic marker in patients with colon cancer because in their study they had found that after treating all the neoplastic specimens of colon with antiprolactin antibody, none of the samples were found to have significantly high pre-operative levels of plasma PRL (26). Baert et al. came to a conclusion that colon cancer does not produce ectopic PRL, but only a subgroup of rectal cancer does. Therefore serum PRL may not be considered as definitive tumor marker in CRC (27).
Alfa feto protein (AFP)
According to Nakagawa et al., CRC cells frequently produce AFP. The postoperative immunohistochemical study revealed AFP production in the cancer tissue. They noticed that chemotherapy with 5-FU reduced the level of AFP in these patients (28). AFP is higher in CRC patients with distant metastasis either in liver or lung. Therefore, AFP could be considered as a potential marker for tumor activity. AFP producing CRC has a very poor prognosis due to frequent occurrence of blood-borne metastases (9). In our study, we also found significantly increased level of AFP among cases, and this was most pronounced in stage IV (patients with distant metastasis). We further found that chemotherapy reduces the level of AFP significantly.
Total human chorionic gonadotropin (hCG)
The presence of hCG in CRC cells has been previously studied, and administration of anti-hCG antiserum caused a significant reduction in this tumor volume (10). Chen noted that hCG is associated with tumour metastasis (11). In a multivariate analysis, independent prognostic significance was observed with hCG beta in CRC (29). However, we could not find any significant increase in the hCG levels in CRC cases compared to controls. Such findings can depend on the sample size, besides genetic and biological variations in patient populations.
Cancer antigen-125 (CA-125)
Streppel et al. found that most of the primary CRCs expressed CA-125 (Mucin16), indicating that it was not only specific for ovarian cancer. CA-125 and its interaction with various receptors might influence cell-cell interactions, allowing cancer cells to evade apoptosis. On mesothelial cells, a binding site, a GPI-anchored glycoprotein with CA-125 was first identified; which mediated cancer cell adhesion and probably played a role in the implantation and metastasis of tumors. Further, an inhibitory receptor for CA-125 was found on immune cells leading to immune response escape. They concluded that CA-125 expression is a late event in the carcinogenesis of digestive tract adenocarcinomas (13). Pre-operative serum CA19-9 (Carbohydrate antigen19-9), CEA and CA-125 can be used an independent prognostic factor for CRC 5-year recurrence-free survival (30). Pre-operative serum CA-125 status and stage were independent prognostic factors for overall survival of CRC (31). In our study we found significantly raised levels of CA-125 in stage IV CRC. Although the overall levels were raised in cases compared to controls, it was not significant. On the other hand, in the post-chemotherapy group the CA-125 levels came down significantly in comparison to pre-chemotherapy conditions.
Testosterone
Lin et al. stated that in male population, lower androgenicity is associated with an increased risk for CRC. It might be due to longer CAG repeats of the androgen receptor (AR) or treatment with androgen deprivation therapy. A second reason might be due to the increased production of estradiol from aromatase conversion which sends a negative feedback response to pituitary that inhibits the secretion of s luteinizing hormone (LH) ultimately leading to decrease in testosterone secretion (14). In our study, we found significantly low level of testosterone among cases in comparison to controls. Although in post-chemotherapy group, we found an increased level of the hormone in comparison to pre-chemotherapy patients, it was not significant. This level also didn’t vary across the stages of CRC.
Prostate specific antigen (PSA)
Prostate specific markers are studied as markers in CRC. According to Haffner et al. prostate-specific membrane (PSM) antigen is expressed in gastric and CRCs (32). The combination of four markers [CEA, f-PSA, CA-125 and carbohydrate antigen (CA)-242] or (CEA, CA19-9, CA125 and f-PSA) has been established as for diagnosis of CRC (15). We failed to find any significant differences in PSA between cases and controls. More studies are required to explored in this regard.
Ferritin
In some studies ferritin has been considered as markers in CRC (15). In CRC due to occult blood loss patients usually suffers from iron deficiency anaemia which might be the reason behind the altered serum ferritin level (33). Chua stated that high dietary iron intake and body iron stores might increase CRC risk. Dietary iron promotes colonic inflammation and tumor development. Chronic inflammation in colon was thought to cause dysregulation of iron metabolism and inhibition of erythropoiesis. The inflammatory cytokine interleukin (IL)-6 increases the synthesis of liver hepcidin, a major iron-regulatory hormone central to iron homeostasis, resulting in reduced duodenal iron absorption and retention of iron in macrophages and hepatocytes promoting iron storage (34). We failed to find any significant differences in ferritin levels between cases and controls in our study. Some retrospective studies for the diagnosis of gastrointestinal cancer also concluded that ferritin has no value as a CRC screening test (33).
Limitations
Our study suffered from limited sample size and inclusion of male sex only. We did not include female patients (pre or post menopausal) since about 95% of the CRC patients attending the clinics are males.
Due to limited resources, we could not measure important parameters like CA-19-9, CA-242, f-PSA, iron and transferrin, which could have provided additional insights. Moreover our study was not powered to study this sub-group analysis as depicted in Table 3.
Conclusions
The present study clearly demonstrates that in north Indian male CRC patients, PRL, AFP, CA-125 and testosterone might be considered as important biomarkers. Furthermore AFP, CA-125 and CEA can be used to assess the effectiveness of chemotherapy. Further prospective studies with analysis of these markers after each cycle of chemotherapy are required to assess their precise prognostic values. Future research may like to explore the use of testosterone therapy as a treatment option in Indian CRC patients; while exploring the use of PRL receptor antagonists might also be interesting.
Acknowledgements
We sincerely acknowledge the help and support received from all technical staffs of Department of Biochemistry, PGIMS, Rohtak, Haryana, India.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Prevention CfDCa. Colorectal (Colon) Cancer. 2010; Available online: http://www.cdc.gov/cancer/colorectal/, accessed 03/02/2014.
- Ferlay J, Soerjomataram I, Ervik M, et al. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 2012 Available online: http://globocan.iarc.fr, accessed 2/2/2014.
- Zeestraten EC, Benard A, Reimers MS, et al. The prognostic value of the apoptosis pathway in colorectal cancer: a review of the literature on biomarkers identified by immunohistochemistry. Biomark Cancer 2013;5:13-29. [PubMed]
- Gür T, Demir H, Kotan MÇ. Tumor markers and biochemical parameters in colon cancer patients before and after chemotherapy. Asian Pac J Cancer Prev 2011;12:3147-50. [PubMed]
- Thomas SN, Zhu F, Schnaar RL, et al. Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E- and L-selectin in shear flow. J Biol Chem 2008;283:15647-55. [PubMed]
- Ben-Jonathan N, Liby K, McFarland M, et al. Prolactin as an autocrine/paracrine growth factor in human cancer. Trends Endocrinol Metab 2002;13:245-50. [PubMed]
- Bhatavdekar JM, Patel DD, Giri DD, et al. Comparison of plasma prolactin and CEA in monitoring patients with adenocarcinoma of colon and rectum. Br J Cancer 1992;66:977-80. [PubMed]
- Otte JM, Otte C, Beckedorf S, et al. Expression of functional prolactin and its receptor in human colorectal cancer. Int J Colorectal Dis 2003;18:86-94. [PubMed]
- Fu K, Kobayashi A, Saito N, et al. Alpha-fetoprotein-producing colon cancer with atypical bulky lymph node metastasis. World J Gastroenterol 2006;12:7715-6. [PubMed]
- Khare P, Singh O, Jain SK, et al. Inhibitory effect of antibodies against human chorionic gonadotropin on the growth of colorectal tumour cells. Indian J Biochem Biophys 2012;49:92-6. [PubMed]
- Chen C, Chen LQ, Yang GL, et al. The application of C12 biochip in the diagnosis and monitoring of colorectal cancer: systematic evaluation and suggestion for improvement. J Postgrad Med 2008;54:186-90. [PubMed]
- Carpelan-Holmström M, Louhimo J, Stenman UH, et al. CEA, CA 242, CA 19-9, CA 72-4 and hCGbeta in the diagnosis of recurrent colorectal cancer. Tumour Biol 2004;25:228-34. [PubMed]
- Streppel MM, Vincent A, Mukherjee R, et al. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol 2012;43:1755-63. [PubMed]
- Lin JH, Zhang SM, Rexrode KM, et al. Association between sex hormones and colorectal cancer risk in men and women. Clin Gastroenterol Hepatol 2013;11:419-424.e1.
- Chen C, Chen LQ, Yang GL, et al. Value of tumor markers in diagnosing and monitoring colorectal cancer and strategies for further improvement: analysis of 130 cases. Ai Zheng 2007;26:1221-6. [PubMed]
- Murray RK, Bender DA, Botham KM, et al. Harpers Illustrated Biochemistry. 28th ed. China: McGraw Hill, 2009.
- Burtis CA, Ashwood ER, Bruns DE. editors. Teitz Textbook of Clinical Chemistry and Molecular Diagnostics. 4th ed. St. Louis: Elsevier, 2006.
- Mayer RJ. Gastrointestinal Tract Cancer. 18th edition ed. New York: McGraw Hill, 2008.
- Bhatavdekar JM, Patel DD, Chikhlikar PR, et al. Ectopic production of prolactin by colorectal adenocarcinoma. Dis Colon Rectum 2001;44:119-27. [PubMed]
- Soroush AR, Zadeh HM, Moemeni M, et al. Plasma prolactin in patients with colorectal cancer. BMC Cancer 2004;4:97. [PubMed]
- Barrera MG, Trejo B, Luna-Péerez P, et al. Opposite association of serum prolactin and survival in patients with colon and rectal carcinomas: influence of preoperative radiotherapy. Dig Dis Sci 2006;51:54-62. [PubMed]
- Ilan Y, Sibirsky O, Livni N, et al. Plasma and tumor prolactin in colorectal cancer patients. Dig Dis Sci 1995;40:2010-5. [PubMed]
- Neradugomma NK, Subramaniam D, Tawfik OW, et al. Prolactin signaling enhances colon cancer stemness by modulating Notch signaling in a Jak2-STAT3/ERK manner. Carcinogenesis 2014;35:795-806. [PubMed]
- Wood AJ, Thomas CM, Baumforth KR, et al. Absence of prolactin gene expression in colorectal cancer. Mol Pathol 1999;52:135-9. [PubMed]
- Carlson HE, Zarrabi MH, Lyubsky SL. Lack of association between hyperprolactinemia and colon carcinoma. Cancer Invest 2000;18:130-4. [PubMed]
- Indinnimeo M, Cicchini C, Memeo L, et al. Plasma and tissue prolactin detection in colon carcinoma. Oncol Rep 2001;8:1351-3. [PubMed]
- Baert D, Matthys C, Gillardin JP, et al. Prolactin and colorectal cancer: is there a connection? Acta Gastroenterol Belg 1998;61:407-9. [PubMed]
- Nakagawa K, Koike S, Matsumura H, et al. alpha-fetoprotein producing rectal cancer. Gan To Kagaku Ryoho 2012;39:671-4. [PubMed]
- Louhimo J, Carpelan-Holmström M, Alfthan H, et al. Serum HCG beta, CA 72-4 and CEA are independent prognostic factors in colorectal cancer. Int J Cancer 2002;101:545-8. [PubMed]
- Yang XQ, Chen C, Wang FB, et al. Preoperative serum carcinoembryonic antigen, carbohydrate antigen19-9 and carbohydrate antigen 125 as prognostic factors for recurrence-free survival in colorectal cancer. Asian Pac J Cancer Prev 2011;12:1251-6. [PubMed]
- Yang XQ, Li Y, Chen C, et al. Preoperative serum carbohydrate antigen 125 level is an independent negative prognostic marker for overall survival in colorectal cancer. Med Oncol 2011;28:789-95. [PubMed]
- Haffner MC, Kronberger IE, Ross JS, et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum Pathol 2009;40:1754-61. [PubMed]
- Baicus C, Caraiola S, Rimbas M, et al. Ferritin above 100 mcg/L could rule out colon cancer, but not gastric or rectal cancer in patients with involuntary weight loss. BMC Gastroenterol 2012;12:86. [PubMed]
- Chua AC, Klopcic BR, Ho DS, et al. Dietary iron enhances colonic inflammation and IL-6/IL-11-Stat3 signaling promoting colonic tumor development in mice. PLoS One 2013;8:e78850. [PubMed]


