The role of head-up cardiopulmonary resuscitation in sudden cardiac arrest: a systematic review and meta-analysis
Introduction
Sudden cardiac arrest (SCA) is the abrupt loss of cardiac activity leading to a lack of systemic perfusion (1), making it the most devastating and time-critical medical emergency. Successful treatment can potentially avert certain death and allow return to an active life in the community. Early and effective cardiopulmonary resuscitation (CPR) is key to achieving good clinical outcomes (2,3). However, clinical outcomes had remained poor in the past 30 years, with out-of-hospital cardiac arrest (OHCA) survival rates ranging from 4.9% to 18.2% (3). Given the large disease burden exerted by SCA (4), there is an urgent need to discover therapeutics to improve clinical outcomes.
Head-up CPR (HU-CPR) is an experimental technique which involves performing high-quality CPR with the patient’s torso and head in an inclined position. There is an expanding body of literature both optimizing the protocol of HU-CPR (e.g., in terms of angle of elevation) and investigating its treatment effects (5,6). It has been purported that HU-CPR improves neurological prognosis in SCA by improving intra-arrest brain perfusion (7). In this postulated mechanism, gravity facilitates drainage of blood from the brain, which lowers intracranial pressure (ICP) and in turn improves cerebral perfusion (7,8). This addresses the unmet need that CPR in its current supine form [hereafter, “conventional or S-CPR)”] is only able to attain up to 30% of both normal cerebral and coronary blood flow (6,7,9). One contributing factor is that during the compression phase, concurrent pressure increases in both the right and left sides of the heart leads to increases in intrathoracic pressure (ITP) and hence ICP, which compromises cerebral perfusion (10,11).
Despite a paucity of randomized human data to elucidate the efficacy or effectiveness of HU-CPR, a few centres have implemented HU-CPR as standard protocol (e.g., Palm Beach County Fire Rescue, Florida, United States and Rialto Fire Department, California, USA), with astounding preliminary clinical results from their observational data (11,12). At the same time, expert recommendations have been made in support of implementing HU-CPR (12). There is an urgent need to consolidate the literature, both preclinical and clinical data, to clarify the role of HU-CPR in SCA.
In this systematic review and meta-analysis, we synthesized the available evidence for the use of HU-CPR in the treatment of cardiac arrest. The primary hypothesis was that HU-CPR improves survival in cardiac arrest compared to S-CPR. The secondary hypothesis was that HU-CPR improves physiological surrogate markers of clinical outcomes as well as the intermediate clinical outcome of return of spontaneous circulation (ROSC). We present the following article in accordance with the PRISMA (13) reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-4984/rc).
Methods
Search strategy
This systematic review has been submitted to PROSPERO (ID: 300352). The search strategy was developed in consultation with a medical information specialist. Employing different keyword combinations [Head up CPR, Head-up CPR, Heads up CPR, Heads-up CPR, resuscitation, CPR, cardiac arrest, cardiopulmonary resuscitation, cardio-pulmonary resuscitation and Chest compress*], a comprehensive search was performed on the bibliometric databases PubMed, Embase and the Cochrane Library from inception to May 1st 2021. The title/abstract screening was performed by two independent reviewers (YKT & AFWH). For articles of interest, full text versions were obtained, with their corresponding reference lists examined for further identification of relevant studies. Any disagreement was resolved by discussion and consensus with a senior author (MEHO).
Study and cohort selection
All study designs (case reports, case series, preclinical studies, randomized controlled trials and observational cohort studies) that reported the use of HU-CPR were included during the initial search. We subsequently excluded all studies that reported on other positions during CPR (such as passive leg raise), studies that did not contain primary data, and those without an English translation.
Data extraction
Relevant quantitative data were extracted by two authors (YKT & AFWH) in the form of absolute frequencies of events or absolute counts when appropriate. We presented continuous variables as mean and standard deviation (SD). Categorical variables were presented as percentages. Where available, the data included several outcome measures of interest: neurologically-intact survival at 24-hour, survival to 24-hour, ROSC, ICP, cerebral perfusion pressure (CerPP) and brain blood flow (BBF).
Risk of bias assessment
The quality and risk of bias of included randomized and non-randomized studies were assessed using the GRADE Assessment Tool (14) and the Newcastle Ottawa Scale (15) respectively. The GRADE Assessment tool assesses quality of evidence in terms of study limitations, inconsistency, indirectness, imprecision and publication bias. The Newcastle Ottawa scale evaluates quality of evidence based on selection of study groups (4 points), comparability of groups (2 points), and ascertainment of exposure and outcomes (3 points). These were graded with the consensus of 3 researchers (AFWH, YKT and MXH).
Statistical analysis
In our meta-analysis, fixed- and random-effects models were used in conjunction with the Sidik-Jonkman estimator and Mantel-Haenszel method to estimate the pooled effects of HU-CPR at 30 degrees depending on the presence of substantial between-study heterogeneity. Studies that examined HU-CPR at 30 degrees inclination were selected to be pooled as that represented the most common intervention among all studies. Forest plots displayed individual and pooled odds ratios (OR) and 95% confidence intervals (95% CI) for binary outcomes. Individual and pooled mean difference (MD) and 95% CI were presented for continuous outcomes. Two-tailed statistical significance was set at P value <0.05. Between-study heterogeneity was assessed using the I2 statistic. Publication bias was assessed using funnel plots if there were 10 or more studies reporting the same outcome. All data analyses were conducted using the Cochrane Collaboration’s Review Manager (RevMan 5.4) Software Package.
Results
Study selection
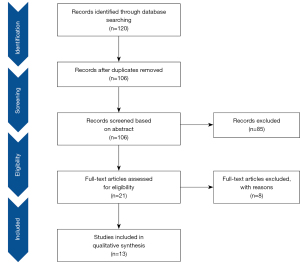
The study identification and selection process were shown in Figure 1. The electronic database search yielded 120 studies, of which 14 studies were removed as duplicates. A further 85 studies were excluded after a screen of title and abstract as they did not report the use of HU-CPR. Then, 8 articles were excluded after full text review. Finally, 13 eligible studies were included in our systematic review and meta-summary (5-8,11,16-23).
Characteristics of included studies
The 13 included studies consisted of only one clinical human-only study, 11 animal-only studies and one study that utilized both human cadavers and animals. A meta-summary of included studies was presented in Table 1 (human and human-cadaveric studies) and Table 2 (animal studies).
Table 1
| Study, year (country) | Study design and sample size (N) | Population/model | Outcome measures | Intervention type and controls for comparison | Results | Conclusions |
|---|---|---|---|---|---|---|
| Moore et al. 2018 (USA) | Experimental trial (N=18); 9 pigs and 9 human cadavers; findings from the porcine protocols are reported in Table 2 | 6 female and 3 male human cadavers with average age 84±10 years, average weight 70±14 kg, average height 1.7±0.1 m, and average interval since death of 3.7±2 days | CerPP; ICP | HC protocol: 6 min of untreated VF; CPR performed for 1 min epochs as follows: standard (S)-CPR supine (SUP), ACD + ITD CPR SUP, then ACD + ITD HUP CPR | Mean CerPP in human cadaver: 1.3±4 for ACD + ITD SUP; 11.3±5 for ACD + ITD HUP (P=0.007); mean ICP was significantly lower in the ACD + ITD HUP group versus the ACD + ITD SUP group in all three CPR models | HU-CPR decreased ICP while increasing CerPP in HC CPR models |
| Mean ICP in human cadaver during compression and decompression: compression: 2.7±3 for ACD + ITD SUP versus −5.4±6 for ACD + ITD HUP (P=0.007); decompression: 0.2±3 for ACD + ITD SUP versus −9.8±8 for ACD + ITD HUP (P=0.007) | ||||||
| Pepe et al. 2019 (USA) | Observational study (N=2,322) | Human, out-of-hospital cardiac arrest (all presenting rhythms) | Primary outcome: resuscitation rate: defined as hospital arrival with ROSC sustained for 5 min; Secondary outcome: intact neurological status (modified Rankin Score <3) |
Pre-intervention: ITD + LUCAS mCPR with pit-crew approach | Mean resuscitation rate pre-intervention vs. post-intervention: 17.87% (range, 14.81–20.13%; n=806) vs. 34.22% (range, 29.76–39.42%; n=1,356, P<0.0001) | Bundled intervention doubled resuscitation rates in out-of-hospital cardiac arrest |
| Additional measures during intervention period: | ||||||
| (I) Delayed positive pressure ventilations after application of oxygen | ||||||
| (II) Strengthening of the team set-up for fast LUCAS placement | ||||||
| (III) Positioning of patient in reverse Trendelenburg (20 degrees) by raising the angle of the whole stretcher, after the placement of LUCAS and advanced airway insertion connection to an ITD |
ICP, intracranial pressure; CerPP, cerebral perfusion pressure; HC, human cadaver; ACD, active compression-decompression device; SUP, standard (S)-CPR supine; HUP, head-up position; ITD, impedance threshold device; LUCAS, chest compression system.
Table 2
| Study, year (country) | Study design and sample size (N) | Species/model | Outcome measures | Intervention type and controls for comparison | Results | Conclusions |
|---|---|---|---|---|---|---|
| Debaty et al. 2015 (USA) | Experimental trial (N=30) | Female Yorkshire farm-bred pigs weighing 39.3±0.5 kg | CoPP, CerPP, ICP, BBF | Preparation: 6 min of untreated VF; 3 min of LUCAS mCPR + ITD in supine position Protocol A: 5 min each of LUCAS mCPR + ITD at 0, 30 deg HUP and 30 deg HDT; 2 min of LUCAS mCPR+ITD at 30 deg HUP; 2 min of LUCAS mCPR at 30 deg HUP Protocol B: interventions as per Protocol A but with microspheres injected before induction of VF and during CPR Protocol C: 1 min each of LUCAS mCPR + ITD at 0, 10, 20, 30, 40 and 50 deg HUP |
CoPP: 19±2 at 0◦ vs. 30±3 at 30◦ HUT (P<0.001); 10±3 at 30◦ HDT (P<0.001) CerPP: 19±3 at 0◦ vs. 35±3 at 30◦ HUT (P<0.001); 4±4 at 30◦ HDT (P<0.001) ICP: with 0, 10, 20, 30, 40 and 50◦ HUT, ICP values were 21±2, 16±2, 10±2, 5±2, 0±2, −5±2 respectively (P<0.001) BBF: 0.19±0.04 mL/min/g at 0◦ vs. 0.27±0.04 at 30◦ HUT (P=0.01); 0.14±0.06 at 30◦ HDT (P=0.16) CSE in Protocol C: CerPP increased linearly while CoPP remained constant |
HUT during LUCAS mCPR + ITD lowered ICP significantly and also improved cerebral perfusion; HDT reduced brain blood flow |
| Duhem et al. 2021 (USA) | Randomised experimental trial (N=15) | Female Yorkshire farm-bred pigs weighing approximately 40 kg | Primary outcome: CerPP | Protocol A: 7.75 min of untreated VF; 30 min of HUP CPR followed by defibrillation and ROSC; 10 min in HUP; randomised to four 5-min epochs of HUP or flat position Protocol B: 6 min of untreated VF6 min of S-CPR followed by defibrillation and ROSC; 10 min in SUP; randomised to four 5-min epochs of HUP or flat position |
ICP: significantly lower after ROSC with HUP position vs. SUP position (9.1±5.5 vs. 18.5±5.1, P<0.001) CerPP: significantly higher after ROSC with HUP position vs. SUP position (62.5±19.9 vs. 53.2±19.1, P=0.004) |
Elevating the head and thorax after ROSC resulted in higher CerPP levels and lower ICP levels in a porcine model of cardiac arrest |
| Kim et al. 2017 (South Korea) | Randomised Experimental Trial (N=12) | Female pigs weighing 42±3 kg |
CerPP; CoPP | Preparation: 6 min of Untreated VF; 3 min of LUCAS mCPR at supine position Intervention: 5 min each of mCPR at three different positions, each with varying angles (I) Head Down Tilt (HDT): −30, −45, −60 degrees; (II) Supine: 0 degrees; (III) Head Up Tilt (HDT): 30, 45, 60 degrees; pigs were randomized to 1 of 2 tilt sequences: HDT Supine HUT or HUT Supine HDT |
CerPP: means (SDs) of CerPP increased consistently; 2.4 (0.4), 9.3 (1.6), 16.5 (1.6), 27.0 (1.5), 35.1 (0.4), 39.4 (0.6), and 39.9 (0.3) mmHg, as angles changed from HDT (−60 degrees) to HUT (60 degrees); CerPPs peaked at HUT 60 degrees CoPP: peaked at HUT 30 degrees ROSC: 100% for all protocols after subjects were defibrillated ICP: means (SDs) of ICP decreased consistently 59 (0.7), 51.3 (1.8), 41.4 (1.2), 27.8 (1.8), 8.9 (0.3), −3.8 (0.5), −7 (0.2) as angles changed from HDT (−60 degrees) to HUT (60 degrees) |
CerPP increased with consistently greater head up position; CoPP was peak at 30 degrees HUP |
| Moore, 2016 (abstract only) (USA) | Randomised experimental trial (N=21); HUP =12; SUP =9 | Pigs | CPC Score at 24-hour; Neurological Deficit Score (NDS) at 24-hour | Preparation: untreated VF for 12 min; ACD + ITD CPR for 1.5 min Control Group: ACD + ITD CPR in SUP position for 6.5 min Experimental Group: ACD + ITD CPR in HUP position for 6.5 min |
Survival to 24-hour: | Higher rate of intact neurological survival for HUP group |
| • HUP: 8/12 | ||||||
| • SUP: 6/9 | ||||||
| CPC ≤2 at 24-hour: | ||||||
| • HUP: 6/12 | ||||||
| • SUP: 3/9 | ||||||
| Mean CPC score at 24-hour: | ||||||
| • HUP: 1.6±0.3 | ||||||
| • SUP: 2.5±0.6 | ||||||
| Mean NDS score at 24-hour: | ||||||
| • HUP: 44±22 | ||||||
| • SUP: 88±45 | ||||||
| Moore et al. 2017 (United States) | Randomised experimental trial (N=18); HUP =8; SUP =10 | Female Yorkshire farm-bred pigs weighing 36–44 kg | Primary outcome: BBF Secondary outcomes: ICP; CerPP |
Preparation: 8 min of untreated VF; 2 min of ACD + ITD CPR in the SUP position Control Group: 18 min of ACD + ITD CPR in SUP position Experimental Group: 18 min of ACD + ITD CPR in 30 degrees HUP |
BBF: 0.42±0.05 HUP (n=8); 0.21±0.04 SUP (n=10) | Brain blood flow was 2-fold higher for ACD + ITD CPR in HUP position versus SUP position Statistically significantly lower ICP and higher CerPP after 5, 15, 19 and 20 min of CPR |
| CerPP at 5, 15, 19, 20 min of CPR: HUP: 26±7; 28±5; 27±5; 20±7 SUP: 13±7; 11±9; 8±10; 6±11 |
||||||
| ICP at 5, 15, 19, 20 min of CPR HUP: 10.0±7.0; 7.7±5.5; 6.1±5.1; 2±2 SUP: 18.3±6.4; 17.7±5.5; 15.7±4.2; 14±2 |
||||||
| ROSC: 5/8 in HUP versus 3/10 in SUP (P=0.34) | ||||||
| Moore et al. 2018 (USA) | Experimental trial (n=18) 9 pigs and 9 human cadavers Findings from the human cadaver protocol are reported in Table 1 |
Female Yorkshire farm-bred pigs weighing 38–42 kg | CerPPICP | Porcine + Porcine Cadaver (PC) Protocol: 6 min of untreated VF CPR was performed for 2 min epochs as follows: standard (S)-CPR supine (SUP), ACD + ITD CPR SUP, then ACD + ITD HUP CPR. The same sequence was performed in PC 3 h later | Mean CerPP in porcine VF: 14.5±6 for ACD + ITD SUP; 28.7±10 for ACD + ITD HUP (P=0.007) Mean CerPP in porcine cadaver: −3.6±5 for ACD + ITD SUP; 7.8±9 for ACD + ITD; HUP (P=0.007) Mean ICP in porcine VF during compression and decompression: compression: 20.6±6 for ACD + ITD SUP versus 13.1±6 for ACD + ITD HUP (P=0.007); decompression: 16.6±5 for ACD + ITD SUP versus 9.8±6 for ACD + ITD HUP (P=0.007) Mean ICP in porcine cadaver during compression and decompression: compression: 12.8±4 for ACD + ITD SUP versus 4.2±3 for ACD + ITD HUP (P=0.007); decompression: 11.9±3 for ACD + ITD SUP versus 3.3±3 for ACD + ITD HUP (P=0.007) |
HUP CPR decreased ICP while increasing CerPP in pigs in VF as well as in PC CPR models |
| Moore et al. 2020 (USA) | Randomised experimental trial (N=30); N=18 for Study A; N=6 for each sequence in Study B | Female Yorkshire farm-bred pigs weighing approximately 40 kg | Primary outcome: CerPP | Preparation: 8 min of untreated VF in pigs Study A: different angles (20, 30, 40 deg) were assessed, each randomized over 5-min periods of ACD + ITD CPR Study B: pigs were randomized to 1 of 2 sequences: 20➝30➝40 or 40➝30➝20 degrees |
CerPP in Study A: equivalent for 30 degrees and 40 degrees; 44±22 and 47±26, P=0.18; significantly higher for 40 degrees than 20 degrees (47±25 versus 38±18, P=0.002) CerPP in Study B at 17 min: higher CerPP in the 20➝30➝40 sequence: 60±17 versus 33±18 (P=0.035) ICP during decompression in Study A: lower for 40 degrees than 20 degrees (6±4 versus 11±4, P value not specified) ICP in Study B at 17 min: lower in the 20➝30➝40 sequence (11±5 versus 16±4, P value not specified) |
No optimal HUP CPR angle was observed. However, controlled progressive elevation of the head and thorax during CPR is more beneficial than an absolute angle or height to maximise CerPP |
| Moore et al. 2021 (USA) | Randomised experimental trial (N=16) | Female Yorkshire farm-bred pigs weighing approximately 40 kg | Primary outcome: Neurologically Intact Survival (CPC Score) Secondary outcome: CoPP |
Preparation: sedation, intubation and anaesthesia followed by 10 min of untreated VF Control Group: conventional CPR (C-CPR) supine position for 19 min Experimental Group: ACD + ITD CSE CPR with various stages; 2 min of ACD + ITD CPR with Customised Elevation Device (CED) in lowest position2 min elevation of CED to highest position; 15 min of ACD + ITD CPR with CED in highest position |
ROSC: 8/8 (100%) with ACD + ITD CSE; 3/8 (25%) for C-CPR (P=0.026) CPC at 24-hour: 6/8 (75%) pigs had a CPC score 1 or 2 with ACD + ITD CSE; 1/8 (12.5%) with C-CPR (P=0.04) CoPP (mean ± SD): significantly higher (41±24) with CSE at 18 min vs. C-CPR (10±5) at 18 min (P=0.004) |
Bundled resuscitation approach of CSE with ACD + ITD CPR increased favourable neurological survival versus C-CPR in a porcine model of cardiac arrest |
| Park et al. 2019 (South Korea) | Randomised experimental trial (n=18) | Female Yorkshire farm-bred pigs weighing 42±3 kg | Primary outcome: 24-hour survival Secondary outcome: ROSC rate after 6 min of BLS |
Preparation: 2 hr of surgical preparation involving sedation, intubation and paralysis; 15 min of untreated VF Control group: 6 min of ACD + ITD CPR in supine position Experimental group: 6 min of ACD + ITD CPR in HUP 30 deg position Post-intervention: defibrillation (if shockable rhythm) at 200 J • if ROSC: additional hydration and adrenaline for up to 90 min • if no ROSC: additional 20 min of ACD + ITD CPR in previous position with adrenaline every 3 min and defibrillation every 2 min |
ROSC: lower in HUP (1/8) vs. SUP (6/8) P=0.04 24-hour survival: 0 in HUP vs. 6/8 in SUP ICP: −4.8±3.1 in HUP vs. 19.7±3.9 in SUP (P<0.01) CerPP: 22.9±7.2 in HUP vs. 17.1±5.0 in SUP (P=0.08) CoPP: 10.6±7.9 in HUP vs. 18.4±11.0 in SUP (P=0.12) |
HUP positioned CPR with a 30 deg angle showed lower rate of survival to 24-hour and lower ROSC rate than CPR in supine position in a porcine cardiac arrest model |
| Putzer et al. 2018 (Austria) | Randomised experimental trial (n=19) | 12- to 16-week-old local pigs, weighing 31–45 kg each |
Primary outcomes: ICP; CerPP Secondary outcomes: rSO2; PbcO2; ScvO2 |
Preparation: 8 min of untreated VF Control group: LUCAS mCPR in SUP Position for 20 min Experimental group: LUCAS mCPR in HUP Position (30 deg) for 20 min |
ICP at 5 min: significantly lower in HUP vs. SUP (18.0±4.5 vs. 24.1±5.2, P=0.033) ICP at 20 min: significantly lower in HUP vs. SUP (12.0±3.4 vs. 17.8±4.3, P=0.023) CerPP at 5 min: significantly higher in HUP vs. SUP (11.2±9.5 vs. 1.0±9.2, P=0.045) CerPP at 20 min: significantly higher in HUP vs. SUP (3.4±6.4 vs. −3.8±2.8, P=0.023) |
HUP did not lead to improvements in cerebral oxygenation or metabolism |
| Rojas-Salvador et al. 2020 (United States) | Randomised experimental trial (n=24) | Female Yorkshire farm-bred pigs weighing approximately 40 kg | Primary outcome: CerPP | Preparation: 8 min of untreated VF; 2 min of automated ACD + ITD CPR Protocol A: ACD + ITD CPR with CSE (to maximum CED height) over either 4 or 10 min Protocol B: ACD + ITD CPR with CSE (to maximum CED height) over 2 min Protocol C: ACD + ITD CPR with CSE (to maximum CED height) over 24 seconds, without initial 2 min of ACD + ITD CPR |
CerPP after 7 min of CPR: significantly higher in 4- vs. 10-min groups in Protocol A (53±14.4 vs. 38.5±3.6 mmHg respectively, P=0.03); significantly higher in 2-min (P=0.031) and 4-min groups (P=0.032) vs. 24-sec group Time to 50% BL CerPP: significantly lower in 4- vs. 10-min group (2.5±1.2 vs. 6±3.1 min, P=0.03) ROSC rate: 100% for all protocols after subjects were defibrillated ICP (when lowered to minimum CED height): significant decrease from supine position to minimum CED position (20.4±1.8 vs. 15.6±1.8 mmHg, P=0.03) |
With CSE and ACD + ITD interventions, CerPP values attained half of baseline values within 2.5 min of CPR; and >80% of baseline values after 7 min of CPR |
| Ryu et al. 2016 (USA) | Randomised experimental trial (n=30) | Female Yorkshire farm-bred pigs weighing 39.3±0.5 kg | Primary outcome: CerPP | Preparation: 8 min of untreated VF Group A (2 arms): 2 min of C-CPR in SUP position; 20 min of C-CPR randomized to either HUP 30 deg or SUP positions Group B (2 arms): 2 min of ACD + ITD CPR in SUP position; 20 min of ACD + ITD CPR randomized to either HUP 30deg or SUP positions |
CerPP in Group A at 22 min: 6±3 in the HUP arm versus −5±3 in the SUP arm (P=0.016) CerPP in Group B at 22 min: 51±8 in HUP arm versus 20±5 in SUP arm (P=0.006) ROSC in Group A: 6/8 ROSC in Group B: 6/8 ICP in Group A during compression and decompression at 22 min: compression: 14±1 in the HUP arm versus 23±1 in the SUP arm (P<0.001); decompression: 12±1 in the HUP arm versus 20±1 in the SUP arm (P<0.001) ICP in Group B during compression and decompression at 22 min: compression: 20±2 in the HUP arm versus 26±2 in the SUP arm (P=0.019); decompression: 15±1 in the HUP arm versus 20±1 in the SUP arm (P<0.001) |
CerPP was significantly improved by the HUP positional intervention in both C-CPR and ACD + ITD CPR in a porcine model of cardiac arrest |
ACD, active compression-decompression device; BBF, brain blood flow; C-CPR, conventional CPR; CED, customised head and thorax elevation device; CerPP, cerebral perfusion pressure; CoPP, coronary perfusion pressure. HUP, head-up position; HUT, head-up tilt; HDT, head-down tilt; ICP, intracranial pressure; ITD, impedance threshold device; LUCAS, chest compression system; mCPR, mechanical CPR; NDS, neurological deficit score; PbcO2, brain tissue oxygen tension; ROSC, return of spontaneous circulation; rSO2, cerebral regional oxygen saturation; ScvO2, cerebral venous oxygen saturation; SUP, supine position.
In terms of study designs, the only human study was an observational before-and-after study, which retrospectively analyzed OHCA cases over 3.5 years, during which the EMS service had implemented HU-CPR as part of their cardiac arrest protocol (11). Specifically, the crew implemented HU-CPR as a reverse Trendelenburg position, as part of a care bundle comprising delayed positive pressure ventilation, ITD and LUCAS mechanical CPR (mCPR).
All 12 animal studies involved porcine models of cardiac arrest where pigs were subjected to a period of untreated VF, which varied from 6 to 15 min across study designs (Table 2).
Regarding experimental interventions and controls, the most common treatment was the bundling of HU-CPR with an active compression-decompression device (ACD) and impedance threshold device (ITD). Across seven animal study protocols, HU-CPR was implemented as a 30-degree upward tilt of the head and thorax (Table 2). Four studies investigated the impact of controlled sequential elevation (CSE), of which one specifically investigated how different time periods of CSE could impact cerebral perfusion (23). The comparators were homogenous across the studies, all of which used the supine position as the main control for comparison. Eleven studies had a proper control arm while two studies used self-controls within their protocol arms, where each animal served as its own control.
In terms of study outcomes, three studies investigated 24-hour survival and neurologically-intact survival after 24-hour. Eleven studies measured cerebral perfusion (CerPP) and of which only two measured BBF via injection of microspheres. Twelve studies investigated HU-CPR and related manoeuvres as pre-ROSC interventions while one study investigated the effect that HU-CPR would have on subjects after ROSC had been achieved.
Risk of bias
Quality of evidence was found to be low to moderate due to inconsistency of outcomes as evaluated by the GRADE framework and shown in Table 3 (14). The lone human study achieved 7 out of a maximum of 9 points on the Newcastle-Ottawa Scale, signifying high quality and low risk of bias for selection.
Table 3
| No. of studies | Certainty assessment | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Events, n | Individuals, n | Rate (95% CI) | ||||
| Survival | ||||||||||||
| 3 | Randomised controlled trial | Not serious | Serious | Not serious | Not serious | Undetected | 13 | 53 | 1.14 (0.04–32.49) | ⨁⨁◯◯ Low | Critical | |
| Cerebral perfusion pressure (assessed with mean difference; scale from: −100 to 100) | ||||||||||||
| 4 | Randomised controlled trial | Not serious | Serious | Not serious | Not serious | Undetected | – | 69 | 14.39 (3.07–25.72) | ⨁⨁⨁◯ Moderate | Critical | |
⨁◯◯◯, very low certainty; ⨁⨁◯◯, low certainty; ⨁⨁⨁◯, moderate certainty; ⨁⨁⨁⨁, high certainty.
Survival
In terms of survival with good neurological status, Moore et al.’s 2016 and 2021 porcine studies (6,18) reported cerebral performance category (CPC) scores assessed at 24-hour post-ROSC. Both studies found that animals subjected to HU-CPR had lower CPC scores and higher rates of favourable neurological survival than the S-CPR arm (6,18).
Pepe et al. 2019’s human study reported that the rates of intact neurological survival (modified Rankin score <3, unspecified time frame), collected only for a subset of patients, were similar to the period before HU-CPR interventions were introduced at 35–40% (11).
In terms of 30-day survival or survival to discharge, none of the included studies reported these outcomes.
In terms of 24-hour survival, a total of 37 subjects across two porcine RCTs (6,18) were assessed based on pooled 24-hour survival outcomes. Meta-analytic estimates for 24-hour survival showed no statistically significant benefit for animals where HU-CPR was conducted in comparison to animals that underwent S-CPR, as shown in Figure 2 (OR =3.93, 95% CI: 0.20–77.08, P=0.37, I2=71%). However, it is worth noting that meta-analytic estimates in Figure 2 showed a trend favouring HU-CPR. There was high between-study heterogeneity (I2=71%).

ROSC
Sustained ROSC of five min with hospital arrival was reported as the primary outcome in Pepe et al.’s human study, which they termed “successful resuscitation” (11). After HU-CPR was introduced, there was a two-fold increase in the rates of successful resuscitation from 17.87% (range, 14.81–20.13%; n=806) to 34.22% (range, 29.76–39.42%; n=1,356, P<0.0001).
Two studies using porcine VF models reported that all subjects achieved ROSC after defibrillation in both intervention and control arms, regardless of the angle of elevation (17,23).
With regards to pooled ROSC outcomes, a total of 50 animal subjects across three porcine RCTs (6,7,19) were assessed. Meta-analytic estimates for ROSC showed no statistically significant benefit for animals where HU-CPR was conducted in comparison to animals that underwent C-CPR, as shown in Figure 3 (OR: 3.63, 95% CI: 0.72–18.39, P=0.12). There was low heterogeneity (I2=28%).

ICP
Consistently across seven animal studies, HU-CPR significantly lowered ICP. Moreover, Moore et al.’s 2018 study demonstrated this finding consistently across human-cadaveric, porcine and porcine-cadaveric models (20).
With regards to the pooled outcome of ICP after 20 min of CPR, a total of 53 animal subjects across three porcine RCTs (19,21,22) were assessed. Meta-analytic estimates for ICP showed a statistically significant benefit in animals where HU-CPR was conducted in comparison to animals that underwent S-CPR, as shown in Figure 4 (MD: −14.08, 95% CI: −23.21 to −4.95, P=0.003). High heterogeneity was reported (I2=97%).

CerPP
Consistently across six animal studies, CerPP was significantly higher with HU-CPR. Moore et al.’s 2018 study demonstrated this finding consistently across human-cadaveric, porcine and porcine-cadaveric models (20). Moore et al.’s 2020 study also specified that it was the 20→30→40 deg sequence in CSE that led to the significant increases in CerPP, which attained doubling of baseline values after 17 min of CPR (5). In addition, Rojas-Salvador et al. 2020 reported that CerPP was significantly higher for CSE over four min as compared to a 10 minute rise (23).
With regards to the pooled outcome of CerPP after 20 min of CPR, a total of 69 animal subjects across four porcine RCTs (7,19,21,22) were assessed. Meta-analytic estimates for CerPP showed a statistically significant benefit for animals where HU-CPR was conducted in comparison to animals that underwent C-CPR, as shown in Figure 5 (MD: 14.39, 95% CI: 3.07–25.72, P=0.01). High heterogeneity was reported (I2=93%).

Despite significant heterogeneity (I2=93%), it is worth noting that all four animal studies in the meta-analysis showed a significant effect favouring HU-CPR.
The animal RCTs assessed in the meta-analyses for ICP and CerPP differed slightly in their methodologies. Three studies allocated 8 min for untreated VF as the baseline, with Park et al. being the only study delaying interventions by 15 min. Physiological parameters were measured regularly throughout the intervention periods, which were 20 min in Moore et al. and Putzer et al., and 22 min in Ryu et al. Park was the only study that measured parameters every minute to a maximum of six min which represented the entirety of their intervention period. The longer delay of treatment and subsequent shorter time period allocated for CPR could account for the absence of statistical significance in Park’s findings, with respect to CerPP. All four RCTs defined HU-CPR as elevation of the head and thorax by a 30-degree angle, with Putzer et al. being the only study that administered compressions without the use of an ITD.
BBF
With regards to BBF, a total of 40 animal subjects from 2 RCTs (16,19) were assessed. Meta-analytic estimates for BBF showed a statistically significant benefit for animals where HU-CPR was conducted in comparison to animals that underwent S-CPR, as shown in Figure 6 (MD: 0.14, 95% CI: 0.02–0.27, P=0.03). High heterogeneity was reported (I2=95%).

Both porcine RCTs used similar time periods for their interventions. Debaty et al. 2015 allocated a total of 19 min for CPR (ACD and ITD) while Moore et al. 2017 allocated 18 min for CPR (LUCAS mCPR and ITD). Microspheres to measure blood flow were injected at four instances in Debaty et al. 2015’s protocol, namely at 5 min prior to VF induction, after four min of CPR, after 1 min of HUT (9 min of CPR) and after 1 min of HDT (14 min of CPR). Moore et al. 2017 measured BBF at two instances post-VF, namely after 5 and 15 min of CPR.
Publication bias
Funnel plots could not be assessed as there were fewer than ten studies reporting each outcome.
Discussion
In this systematic review and meta-analysis, several main findings emerged: (I) despite numerous porcine studies on HU-CPR, the only human data came from a single observational study, which reported doubling of ROSC rates; (II) there was overall benefit to neurological outcomes and 24-hour survival in animal subjects, although statistically insignificant, (III) there were statistically significant beneficial pooled effects on ICP, CerPP and BBF in animal subjects. This was to our knowledge the first systematic review and meta-analysis on the role of HU-CPR in SCA.
In terms of patient-oriented outcomes, HU-CPR appeared to improve neurological parameters in both animal studies designed to examine this outcome. This outcome is highly clinically meaningful as it portends quality survivorship after SCA and potentially allows return to an active life in the community. This result is promising as the main purpose of HU-CPR is to optimize cerebral resuscitation. It was noted that no study reported 30-day-survival or survival to discharge, which were outcomes recommended to be reported under the Utstein style for SCA research in general (24). The pooled effect on 24-hour survival was not statistically significant (P=0.37). However, the moderate heterogeneity (I2=71%) limited inference on the true magnitude of effect, and is possibly related to variations in HU-CPR protocol, such as differences between the types of study control used.
Further, we found that HU-CPR showed benefit on the intermediate clinical outcome of ROSC, in the single human study as well as the pooled effect in three animal studies. In addition, there were significant pooled benefits on the physiological parameters of ICP, CerPP and BBF. While Pepe et al.’s human study showed a dramatic doubling of ROSC rate after implementation of HU-CPR, it was unclear how much (if any) of the treatment effect was attributable to HU-CPR. This was because other components of the intervention bundle (ITD, delayed positive pressure ventilation and mCPR) could improve ROSC rate independently (25,26). In the three animal studies pooled, the outcome ROSC, despite being statistically significant, is less clinically important because the determinants of ROSC in experimental induced-VF models were probably different from that in real-life.
Of note, Park et al. was the only study that reported a significantly worse rate of ROSC and 24-hour survival. The reason for this anomaly was unknown, but was possibly related to protocol design. Importantly, the studies’ protocols differed in the length of time pigs were left untreated after inducing VF. While Moore et al.’s protocols subjected pigs to 10–12 min of untreated VF, Park et al. used 15 min. Across all other included studies, the period of untreated VF ranged from 6 to 8 min. This additional delay to HU-CPR could have impacted on haemodynamic parameters and therefore reduced survival rate (27,28). The duration of untreated VF is a possible effect modifier of the benefit of HU-CPR, and hence a possible source of clinical heterogeneity in our study. It is also important to note that Park et al. was the only study that did not prime the pump before doing HUCPR compared to other studies, lacking a suction cup to allow for passive recoil. Not priming the pump could have thus affected the rate of ROSC and survival rate, as shown in other studies (29). The speculation that the benefit of HU-CPR was limited to patients with short downtime is hypothesis generating.
Besides simply ascertaining the efficacy of HU-CPR, a few studies examined additional research questions: (I) interaction effects with ACD and ITD; (II) optimal angle of inclination; (III) CSE. Firstly, some included studies found a positive interaction between HU-CPR and ACD/ITD (6,11,19,20). The ACD is a suction cup integrated into the piston of the mCPR device, which, exerts an active decompressive force after each compression (9,19). The ITD attaches to the airway adjunct and lowers ITP by preventing passive gas exchange during chest wall recoil (9). Figure 7 (11) shows that the effect of ACD/ITD and HU-CPR on CerPP were more than additive. In addition, the inclusion of ACD/ITD to HU-CPR prevented a downward decay in CerPP over time, as compared to HU-CPR with solely a mechanical compression device. It is also important to note that Putzer et al. 2018, as the only study which did not utilise ITD, had demonstrably worse outcomes compared to the rest of the other studies included in the forest plots (Figures 4,5).

Secondly, some included animal studies investigated the optimal angle of elevation. Kim et al. 2017 found that coronary perfusion pressure (CoPP) peaked at 30 degrees, similar to CerPP which rose linearly until 30 degrees and thereafter plateaued (17). Debaty et al. 2015 concluded that while the optimal HUT angle is unknown, it demonstrated that a head-down tilt reduced BBF (16). While the pooled effect of 30-degree inclination was beneficial, these were all found in porcine models and may not be directly translatable to humans.
Thirdly, a few studies implemented a CSE protocol. This meant a sequential elevation of the head and thorax from smaller to larger angles over a specified time frame. Moore et al.’s 2020 study found that CerPP was highest when HUT was increased sequentially from 20 to 40 degrees (5,17). Specifically, it was a 2-minute sequential elevation that produced the most favourable neurological outcome, as reported in Moore et al.’s 2021 study. It was assumed that CSE augmented right to left pulmonary flow and improved autoregulation of systemic vasculature (5).
An additional variation in HU-CPR protocol is of interest. The configuration of a full-body tilt (reverse Trendelenburg) as compared to a head-and-thorax-only (above waist) tilt warrants further deliberation. It was suggested that a full-body tilt leads to greater pooling of blood in the lower extremities, which worsens brain perfusion (19). This might be supported by Pepe et al. 2019’s findings that HU-CPR delivered as a reverse Trendelenburg position resulted in similar rates of neurologically intact survival pre- and post-intervention (11). Ryu et al. 2016’s study had instead created and implemented a head-up device that tilts just the head and upper thorax, resulting in higher CerPP over a 22-min period of ACD + ITD HU-CPR (7). This suggests that elevation of the head and thorax could be preferable over a full-body tilt in HU-CPR.
The transferability of experimental findings from healthy, young pigs to real-life human SCA is a common concern across the included studies. While porcine models of cardiac arrest had been extensively developed and used in SCA research over decades, they were not without limitations (30). For example, the lower limbs of swine differ significantly from human equivalents in terms of the smaller blood volume. A systematic review of 490 studies employing animal models of cardiac arrest revealed that swine were most commonly used due their advantages of similarities to human cardiovascular and neurological physiology (31). Porcine models hence have higher fidelity than rodent models which were less preferred due to higher heart and respiratory rates which complicated compression-ventilation timings (31). While primate models could be superior to porcine models, there is limited collective experience with it in SCA research, where it has only been successfully reported in the field of cardiac xenotransplantation (32).
The accuracy of CerPP, ICP, BBF and rSO2 as surrogates for predicting neurological outcomes should also be evaluated. The studies included in this review reported their findings based on these parameters: CerPP (nine studies), ICP (ten studies), BBF (two studies), rSO2 (one study). In cardiac arrest, cerebrovascular homeostasis is disrupted due to primary ischemia, leading to cerebral oedema and increased ICP, which in turn decreases cerebral blood flow (33,34). Upon ROSC, hypoxic ischaemic encephalopathy, also termed as global ischaemia-reperfusion injury, interferes with cerebral blood flow and perfusion (35-37). CPP may therefore become dependent upon blood pressure or MAP (35). Considering this pathophysiology, it seems valid for CerPP, ICP and BBF to be used as surrogate markers of neurological outcomes. Specifically, BBF has been used to prognosticate neurological status post-cardiac arrest and to inform research in targeted temperature management (37). On the other hand, rSO2 has limited predictive potential for neurological outcomes after SCA (38,39).
There was substantial uncertainty over whether these laboratory findings can be replicated in real life, due to implementation challenges. Firstly, it is generally difficult to implement intra-arrest interventions, due to cardiac arrest care being already complex and the pre-hospital care environment already chaotic. The value of the novel intervention of HU-CPR is present only if the rudimentary criteria of high-quality chest compressions are met (10,12,40). Incremental encumbrance of paramedic resuscitative workflow during SCA may lead to compromise in the quality of key interventional processes (e.g., reducing interruption in CPR and early defibrillation) (12,40). Furthermore, given that there were suggestions that the benefit of HU-CPR was attenuated (or even becomes harmful) when HU-CPR is instituted late, this becomes an implementation challenge because the ambulance response time in many health systems can be very variable (41,42). In addition, the ability to maintain a HU-CPR position while navigating tight urban spaces and bumpy road conditions is yet another challenge. A possible solution is the EleGARDTM device which has been mentioned in some literature (23,43), although further research into its specific use in human models of cardiac arrest is needed.
Limitations
The limitations of this systematic review and meta-analysis should be acknowledged. Firstly, the findings from this report are informed by a predominance of animal studies and hence limited by the paucity of randomized human data. The only observational human study had adopted a pre-post implementation design, which has inherent causal limitations due to the lack of a contemporaneous control group. Further, that study examined HU-CPR as part of a bundle of multiple interventions, which means it is difficult to infer the treatment effect attributable to HU-CPR alone. Secondly, further research is needed to examine the use of HU-CPR with manual hands-only compressions since the findings of this review are limited to CPR conducted with ACD, ITD and mechanical compression devices. Manual hands-only compressions could be challenging to perform in a head-up position. Thirdly, although the use of porcine VF models is common, key anatomical differences remain. Moreover, the swine used in porcine studies were young and healthy, which is not representative of cardiac arrest patients who are likely to present with multiple comorbidities. Fourthly, while CerPP, ICP and BBF have been consistently used as surrogate parameters for neurological outcomes, their accuracy requires future corroboration by future research. Finally, the benefits of HU-CPR in the real-world are dependent heavily on the dynamic and unpredictable nature of the pre-hospital environment and its influence on cardiac arrest management. The certainty of its actual benefit upon implementation is therefore intrinsically linked with paramedic competencies and team cohesiveness during resuscitation, factors which are bound to vary across EMS systems and geographical contexts.
Conclusions
There was an absence of human experimental trials. Overall, HU-CPR improved neurologically-intact survival at 24-hour, ROSC and physiological surrogate outcomes in animal models. Despite promising preclinical data, and one human observational study, clinical equipoise remains surrounding the role of HU-CPR in SCA, necessitating clarification with future randomized human trials.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4984/rc
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4984/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-4984/coif). Dr. DJH is supported by the Duke-NUS Signature Research Programme funded by the Ministry of Health, Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017), Centre Grant (CGAug16M006), and Collaborative Centre Grant scheme (NMRC/CGAug16C006). Dr. AFWH was supported by the Estate of TSKT Puat (Khoo Clinical Scholars Programme), Khoo Pilot Award (KP/2019/0034), and Duke-NUS Medical School and National Medical Research Council (NMRC/CS_Seedfd/012/2018). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Myat A, Song KJ, Rea T. Out-of-hospital cardiac arrest: current concepts. Lancet 2018;391:970-9. [Crossref] [PubMed]
- Ong MEH, Perkins GD, Cariou A. Out-of-hospital cardiac arrest: prehospital management. Lancet 2018;391:980-8. [Crossref] [PubMed]
- Sasson C, Rogers MA, Dahl J, et al. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2010;3:63-81. [Crossref] [PubMed]
- Coute RA, Nathanson BH, Panchal AR, et al. Disability-Adjusted Life Years Following Adult Out-of-Hospital Cardiac Arrest in the United States. Circ Cardiovasc Qual Outcomes 2019;12:e004677. [Crossref] [PubMed]
- Moore JC, Salverda B, Lick M, et al. Controlled progressive elevation rather than an optimal angle maximizes cerebral perfusion pressure during head up CPR in a swine model of cardiac arrest. Resuscitation 2020;150:23-8. [Crossref] [PubMed]
- Moore JC, Salverda B, Rojas-Salvador C, et al. Controlled sequential elevation of the head and thorax combined with active compression decompression cardiopulmonary resuscitation and an impedance threshold device improves neurological survival in a porcine model of cardiac arrest. Resuscitation 2021;158:220-7. [Crossref] [PubMed]
- Ryu HH, Moore JC, Yannopoulos D, et al. The Effect of Head Up Cardiopulmonary Resuscitation on Cerebral and Systemic Hemodynamics. Resuscitation 2016;102:29-34. [Crossref] [PubMed]
- Duhem H, Moore JC, Rojas-Salvador C, et al. Improving post-cardiac arrest cerebral perfusion pressure by elevating the head and thorax. Resuscitation 2021;159:45-53. [Crossref] [PubMed]
- Aufderheide TP, Frascone RJ, Wayne MA, et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial. Lancet 2011;377:301-11. [Crossref] [PubMed]
- Lurie KG, Nemergut EC, Yannopoulos D, et al. The Physiology of Cardiopulmonary Resuscitation. Anesth Analg 2016;122:767-83. [Crossref] [PubMed]
- Pepe PE, Scheppke KA, Antevy PM, et al. Confirming the Clinical Safety and Feasibility of a Bundled Methodology to Improve Cardiopulmonary Resuscitation Involving a Head-Up/Torso-Up Chest Compression Technique. Crit Care Med 2019;47:449-55. [Crossref] [PubMed]
- Pepe PE, Aufderheide TP, Lamhaut L, et al. Rationale and Strategies for Development of an Optimal Bundle of Management for Cardiac Arrest. Crit Care Explor 2020;2:e0214. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021;10:89. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Crossref] [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/ oxford.htm
- Debaty G, Shin SD, Metzger A, et al. Tilting for perfusion: head-up position during cardiopulmonary resuscitation improves brain flow in a porcine model of cardiac arrest. Resuscitation 2015;87:38-43. [Crossref] [PubMed]
- Kim T, Shin SD, Song KJ, et al. The effect of resuscitation position on cerebral and coronary perfusion pressure during mechanical cardiopulmonary resuscitation in porcine cardiac arrest model. Resuscitation 2017;113:101-7. [Crossref] [PubMed]
- Moore J, Ryu H, Robinson A, et al. Neurologic and Hemodynamic Outcomes in a Head Up Versus Supine CPR Survival Model of Porcine Cardiac Arrest: 206. Academic Emergency Medicine 2016;23:
- Moore JC, Segal N, Lick MC, et al. Head and thorax elevation during active compression decompression cardiopulmonary resuscitation with an impedance threshold device improves cerebral perfusion in a swine model of prolonged cardiac arrest. Resuscitation 2017;121:195-200. [Crossref] [PubMed]
- Moore JC, Holley J, Segal N, et al. Consistent head up cardiopulmonary resuscitation haemodynamics are observed across porcine and human cadaver translational models. Resuscitation 2018;132:133-9. [Crossref] [PubMed]
- Park YJ, Hong KJ, Shin SD, et al. Worsened survival in the head-up tilt position cardiopulmonary resuscitation in a porcine cardiac arrest model. Clin Exp Emerg Med 2019;6:250-6. [Crossref] [PubMed]
- Putzer G, Braun P, Martini J, et al. Effects of head-up vs. supine CPR on cerebral oxygenation and cerebral metabolism - a prospective, randomized porcine study. Resuscitation 2018;128:51-5. [Crossref] [PubMed]
- Rojas-Salvador C, Moore JC, Salverda B, et al. Effect of controlled sequential elevation timing of the head and thorax during cardiopulmonary resuscitation on cerebral perfusion pressures in a porcine model of cardiac arrest. Resuscitation 2020;149:162-9. [Crossref] [PubMed]
- Perkins GD, Jacobs IG, Nadkarni VM, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Circulation 2015;132:1286-300. [Crossref] [PubMed]
- Holmberg MJ, Nicholson T, Nolan JP, et al. Oxygenation and ventilation targets after cardiac arrest: A systematic review and meta-analysis. Resuscitation 2020;152:107-15. [Crossref] [PubMed]
- Pitts S, Kellermann AL. Hyperventilation during cardiac arrest. Lancet 2004;364:313-5. [Crossref] [PubMed]
- Perkins GD, Lall R, Quinn T, et al. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised controlled trial. Lancet 2015;385:947-55. [Crossref] [PubMed]
- Wik L, Hansen TB, Fylling F, et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation: a randomized trial. JAMA 2003;289:1389-95. [Crossref] [PubMed]
- Babbs CF, Weaver JC, Ralston SH, et al. Cardiac, thoracic, and abdominal pump mechanisms in cardiopulmonary resuscitation: studies in an electrical model of the circulation. Am J Emerg Med 1984;2:299-308. [Crossref] [PubMed]
- Debaty G, Lurie K, Metzger A, et al. Reperfusion injury protection during Basic Life Support improves circulation and survival outcomes in a porcine model of prolonged cardiac arrest. Resuscitation 2016;105:29-35. [Crossref] [PubMed]
- Vognsen M, Fabian-Jessing BK, Secher N, et al. Contemporary animal models of cardiac arrest: A systematic review. Resuscitation 2017;113:115-23. [Crossref] [PubMed]
- Goerlich CE, DiChiacchio L, Zhang T, et al. Heterotopic Porcine Cardiac Xenotransplantation in the Intra-Abdominal Position in a Non-Human Primate Model. Sci Rep 2020;10:10709. [Crossref] [PubMed]
- Hifumi T, Kawakita K, Yoda T, et al. Association of brain metabolites with blood lactate and glucose levels with respect to neurological outcomes after out-of-hospital cardiac arrest: A preliminary microdialysis study. Resuscitation 2017;110:26-31. [Crossref] [PubMed]
- Kang C, Min JH, Park JS, et al. Relationship between optic nerve sheath diameter measured by magnetic resonance imaging, intracranial pressure, and neurological outcome in cardiac arrest survivors who underwent targeted temperature management. Resuscitation 2019;145:43-9. [Crossref] [PubMed]
- Jakkula P, Pettilä V, Skrifvars MB, et al. Targeting low-normal or high-normal mean arterial pressure after cardiac arrest and resuscitation: a randomised pilot trial. Intensive Care Med 2018;44:2091-2101. [Crossref] [PubMed]
- Huang CH, Tsai MS, Ong HN, et al. Association of hemodynamic variables with in-hospital mortality and favorable neurological outcomes in post-cardiac arrest care with targeted temperature management. Resuscitation 2017;120:146-52. [Crossref] [PubMed]
- Wang Q, Miao P, Modi HR, et al. Therapeutic hypothermia promotes cerebral blood flow recovery and brain homeostasis after resuscitation from cardiac arrest in a rat model. J Cereb Blood Flow Metab 2019;39:1961-73. [Crossref] [PubMed]
- Storm C, Leithner C, Krannich A, et al. Regional cerebral oxygen saturation after cardiac arrest in 60 patients--a prospective outcome study. Resuscitation 2014;85:1037-41. [Crossref] [PubMed]
- Joo WJ, Ide K, Nishiyama K, et al. Prediction of the neurological outcome using regional cerebral oxygen saturation in patients with extracorporeal cardiopulmonary resuscitation after out-of-hospital cardiac arrest: a multicenter retrospective cohort study. Acute Med Surg 2020;7:e491. [Crossref] [PubMed]
- Yannopoulos D, Aufderheide TP, Abella BS, et al. Quality of CPR: An important effect modifier in cardiac arrest clinical outcomes and intervention effectiveness trials. Resuscitation 2015;94:106-13. [Crossref] [PubMed]
- Zhan L, Yang LJ, Huang Y, et al. Continuous chest compression versus interrupted chest compression for cardiopulmonary resuscitation of non-asphyxial out-of-hospital cardiac arrest. Cochrane Database Syst Rev 2017;3:CD010134. [Crossref] [PubMed]
- Grunau B, Kawano T, Scheuermeyer F, et al. Early advanced life support attendance is associated with improved survival and neurologic outcomes after non-traumatic out-of-hospital cardiac arrest in a tiered prehospital response system. Resuscitation 2019;135:137-44. [Crossref] [PubMed]
- Holley J, Moore JC, Jacobs M, et al. Supraglottic airway devices variably develop negative intrathoracic pressures: A prospective cross-over study of cardiopulmonary resuscitation in human cadavers. Resuscitation 2020;148:32-8. [Crossref] [PubMed]