
Exploring the mechanisms of action of Cordyceps sinensis for the treatment of depression using network pharmacology and molecular docking
Introduction
Depression is a chronic mood disorder with a very high prevalence. It can be disabling for patients, who often experience a high incidence of suicide and relapse. Unfortunately, the curative rates for patients with depression are relatively low (1). The clinical manifestations of depression include apathy, loss of interest or pleasure in activities, and slowness in mental and physical activities (2). The etiology of depression is complex, and is often an interplay between genetic and environmental factors, as well as biological, social, and psychological factors (3). Sociologically, it is believed that the prevalence of depression also varies considerably between different ethnic groups and races (4). Psychologically, it is thought that adverse life events may increase the risk of depressive episodes (5). Furthermore, depression is associated with a variety of diseases, such as gastrointestinal disorders, sleep disorders, and neurological disorders (6-8). However, the antidepressants currently available are ineffective and have significant side effects (9). For example, tricyclic antidepressants (TCAs) regulate the concentration of monoamine neurotransmitters in the synaptic gap mainly by inhibiting the reuptake of synaptic gap 5-HT and NE. Common adverse effects are blurred vision, dry mouth, constipation, and in severe cases, urinary retention and intestinal paralysis. Monoamine oxidase inhibitors (MAOIs) are the first class of drugs used to treat depression. They produce antidepressant effects by inhibiting monoamine oxidase (MAO), reducing the metabolic inactivation of catecholamines, and increasing catecholamine content at synaptic sites, however, they have low potency, high side effects, and hepatotoxicity. Therefore, further research and development of antidepressant drugs is crucial.
Traditional Chinese medicine (TCM) and herbal medicines have been widely used for thousands of years to treat a wide variety of diseases (10). TCM is mainly derived from natural plants, which has the advantages of good efficacy, low toxic side effects, and low costs (11). However, the complexity of the components involved in TCM and the unclear mechanisms of action have hampered its clinical application (12). Cordyceps sinensis (BerK.) Sacc. is a complex of the ascospores of the Cordyceps sinensis (BerK.) parasite that is found on the larvae of insects from the Hepialidae family and the bodies of the larvae and is primarily used to treat fatigue, night sweats and other symptoms related to aging (13). It is mainly found in high altitude areas around 4,000 meters above sea level in Qinghai, Tibet, Sichuan, Yunnan, Guizhou, and Gansu in China. It is believed to be a good tonic for the kidneys, beneficial for the lung, and can stopping bleeding and resolve phlegm buildup (14). In recent years, most of the research on Cordyceps sinensis has focused on the chemical composition and pharmacological activities such as the treatment of diabetic nephropathy. However, less research has been conducted on its antidepressant effects and mechanisms (15) and warrant further investigation.
With the development of computer bioinformatics, network pharmacology has gradually become an emerging field. Network pharmacology is based on the principle of systems biology to construct biological networks for elucidating the potential mechanisms of drug therapy for complex diseases, while molecular docking is one of the common methods to study the interaction pattern between small molecules and large molecules, and the recognition between biomolecules. Since Chinese medicine is characterized by multi-components and multi-targets, network pharmacology allows us to effectively connect Chinese medicine to its components, targets, pathways, and diseases. This study examined the potential mechanisms of the antidepressant effects of Cordyceps sinensis by using network pharmacology and molecular docking techniques (Figure 1). We present the following article in accordance with the TRIPOD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-762/rc).
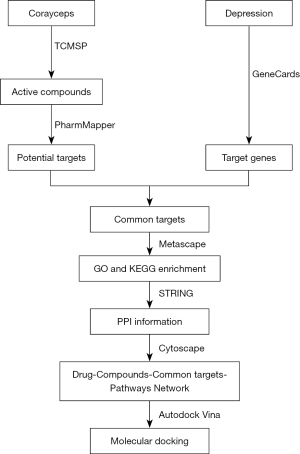
Methods
Identifying the active ingredients of Cordyceps sinensis
The active ingredients of Cordyceps sinensis were assessed using the Traditional Chinese Medicine Systematic Pharmacology Database and Analysis Platform (TCMSP, http://tcmspw.com/tcmsp.php) and 499 herbs with a total of 12,144 chemical substances were identified (16). The search was performed using “Corayceps” as the keyword, and oral bioavailability (OB) ≥30% and drug-likeness (DL) ≥0.18 were applied as the screening conditions to obtain the eligible active ingredients.
Predicting the targets of the active ingredients
Target prediction of all active ingredients was performed through the PharmMapper (http://www.lilab-ecust.cn/pharmmapper) platform, which is supported by the TargetBank, DrugBank, Binding DB, and PDTD databases with over 7,000 receptor-based pharmacological models (17-19). By submitting the active ingredient in mol2 or sdf format on this platform and selecting its default option in the parameter settings, the target information of the active ingredient was generated.
Determining the targets of Cordyceps sinensis in depression
The GeneCards (https://www.genecards.org) database was searched and the target genes for depression were determined by taking the median value of triplicate readings (20). Subsequently, the potential targets of the drug active ingredients were converted into Gene Symbol through the Uniprot (https://www.uniprot.org) platform (21). Finally, the overlapping targets of the Cordyceps sinensis active ingredients and depression were screened and identified. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Enrichment analysis
To determine the biological functions and signaling pathways involved in the common targets of the drug active ingredients and depression, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed using the Metascape (https://metascape.org) platform (22). A P value ≤0.05 for GO and P≤0.05 for KEGG pathway were considered significantly enriched. The results of the enrichment analyses were plotted using http://www.bioinformatics.com.cn, a free online platform for data analysis and visualization.
Construction of a drug, components, targets (including protein-protein interaction), pathway network
The STRING (https://string-db.org) database is a platform used to predict protein-protein interaction (PPI) networks (23). This platform was used to submit the common targets of the active ingredients of Cordyceps sinensis and depression to obtain PPI network maps and information. Based on the active ingredients, common targets, and signaling pathways, a network model of drug, components, targets, signaling pathways, and diseases was constructed with the Cytoscape 3.9.0 software (24). Drug, components, targets, pathways, and diseases are represented as nodes, and their interactions are represented as edge connections.
Molecular docking
The top 10% of targets from the above network were selected as receptors and their structures were obtained from the Uniprot platform. The active ingredients that can bind to these targets were used as ligands. Molecular docking of the ligand to the receptor was performed using Autodock Vina software (25), and the conformation and minimum binding energy of the ligand to the receptor were obtained after the docking was completed. Subsequently, their binding images were plotted in the pymol software (26).
Statistical analysis
Data processing was performed using GraphPad Prism8.0, protein-protein interaction analysis was performed using Cytoscape 3.9.0, and pathway enrichment analysis was performed by Metascape version 3.5. Molecular docking analysis of proteins and active ingredients was performed using Autodock Vina version 1.2.2. The results of all relevant data analysis are presented in the Result section below.
Results
The active ingredients in Cordyceps sinensis
A total of 7 active ingredients in Cordyceps sinensis were identified using the TCMSP database, namely, arachidonic acid, linoleyl acetate, beta-sitosterol, peroxyergosterol, cerevisterol, cholesteryl palmitate, and cholesterol (CLR). The specific characteristics of these active ingredients are shown in Table 1.
Table 1
| Molecule ID | Molecule name | MW | OB (%) | Caco-2 | BBB | DL |
|---|---|---|---|---|---|---|
| MOL001439 | Arachidonic acid | 304.52 | 45.57 | 1.2 | 0.58 | 0.2 |
| MOL001645 | Linoleyl acetate | 308.56 | 42.1 | 1.36 | 1.08 | 0.2 |
| MOL000358 | Beta-sitosterol | 414.79 | 36.91 | 1.32 | 0.99 | 0.75 |
| MOL011169 | Peroxyergosterol | 428.72 | 44.39 | 0.86 | 0.43 | 0.82 |
| MOL008998 | Cerevisterol | 432.76 | 39.52 | 0.35 | −0.29 | 0.77 |
| MOL008999 | Cholesteryl palmitate | 625.19 | 31.05 | 1.45 | 0.68 | 0.45 |
| MOL000953 | CLR | 386.73 | 37.87 | 1.43 | 1.13 | 0.68 |
MW, molecular weight; OB, oral bioavailability; Caco-2, ingredients’ transport rates (nm/s) in Caco-2 monolayers; BBB, blood-brain barrier; DL, drug-likeness; CLR, cholesterol.
Common targets of the active ingredients and depression
The top 300 targets for each active ingredient were identified using the PharmMapper platform. The GeneCards database was used to obtain 12,902 target genes associated with depression, and 1,525 target genes were obtained by taking the median number three times. By taking the intersection of the active ingredient targets and the disease-related targets, a total of 41 common targets were obtained (Figure 2), and the specific information of these targets is shown in Table 2.
Table 2
| Disease | Compound | Common targets |
|---|---|---|
| Depression | MOL008998 | MAOB, DLG1, CREBBP, AR, HSD11B1, DDC, ESR2, CAT, SOD2, USH1C, F2, HSD17B4, CD8A, BCL2A1, GDI1, NPC2, MMP2, ALAD, SMS, RBP4 |
| MOL000953 | CREBBP, AR, HSD11B1, ACHE, CAT, EGF, SOD2, F2, HSD17B4, CD8A, GDI1, MOG, NPC2, RBP4, ACTA1, HEXA | |
| MOL000358 | MAOB, CREBBP, AR, ACHE, USP8, EGF, SOD2, F2, GDI1, NPC2, RBP4, HEXA | |
| MOL001645 | GAD1, DLG1, S100B, CREBBP, EP300, AR, ACHE, DDC, ZEB2, ESR2, CAT, SOD2, USH1C, F2, HSD17B4, INSR, CD8A, BCL2A1, CDH11, GDI1, MOG, SIN3A, NPC2, ALAD, RBP4, ACTA1 | |
| MOL011169 | DLG1, CREBBP, AR, ACHE, ESR2, CAT, SPD2, HSD17B4, MANB1, CDH11, GDI1, MOG, NPC2, RBP4 | |
| MOL001439 | GAD1, MAOB, DLG1, S100B, CREBBP, AR, DDC, ZEB2, ESR2, CAT, SOD2, USH1C, F2, HSD17B4, CD8A, GDI1, MOG, SIN3A, NPC2, HBB, MMP2, ALAD, RBP4, ACTA1, ANXA5 | |
| MOL008999 | ESR1, GAD1, S100B, CREBBP, EP300, AR, ACHE, DDC, ESR2, SYNGAP1, CAT, SOD2, F2, HSD17B4, VDR, CD8A, HMOX1, GDI1, MOG, NPC2, HBB, CNP, RBP4, ACTA1 |
Enrichment analysis of KEGG pathways and GO
The common targets were uploaded to the Metascape platform and the P value was set to <0.05. Enrichment analyses of KEGG pathways, GO biological processes, GO cellular components, and GO molecular functions were performed. A total of 388 GO biological processes (GO BP), 36 GO cellular components (GO CC), 36 GO molecular functions (GO MF), and 23 KEGG pathways were identified.
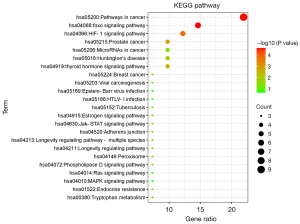
KEGG pathway analysis
All KEGG pathways were selected for analysis and mapping (Figure 3). The potential targets of Cordyceps sinensis activity for the treatment of depression were mainly enriched in foxo signaling pathway (hsa04068), HIF-1 signaling pathway (hsa04066), and Huntington’s disease (hsa05016). These results suggested that the active ingredients of Cordyceps sinensis may exert antidepressant effects by regulating the CREB binding protein and catalase (CAT).
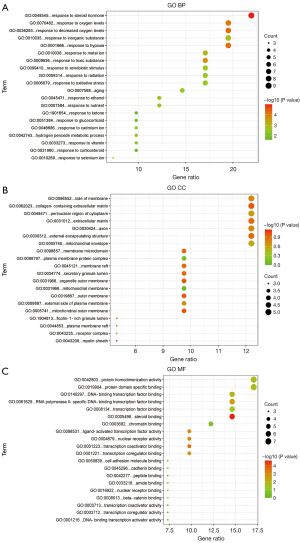
GO analysis
The top 20 items of the GO biological processes, GO cellular components, and GO molecular functions enrichment analyses were further examined (Figure 4A-4C). The potential targets of the active ingredients of Cordyceps sinensis for the treatment of depression are mainly involved in steroid hormone response and anti-oxidative stress response.
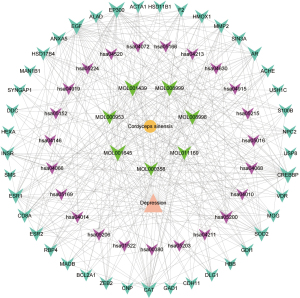
Network analysis of drug-component-target (including PPI)-pathway
By submitting the targets in the STRING database, the PPI interactions network information was obtained, and the network diagram of drug-component-target (including PPI)-pathway was obtained in Cytoscape 3.9.0 by collating the data, as shown in Figure 5. There were 73 nodes (disease: 1 node; drug: 1 node; active ingredients: 7 nodes; targets: 41 nodes; and pathways: 23 nodes) and 353 edges in the network (137 for ingredient-target connections, 84 for PPI, 84 for pathway-target connections, 41 for disease-target connections, and 7 for drug-active ingredient connections).
Molecular docking
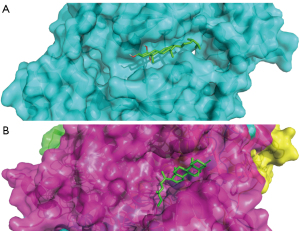
The top 10% of targets in the network (including CREBBP, EP300, EGF, and CAT) were docked to the relevant active ingredients using Autodock Vina. The results showed a total of 17 binding interactions, with all molecules showing a binding energy less than 0 with the targets (Table 3), suggesting that the ligand can spontaneously bind to the receptor. Figure 6A shows the docking results of cerevisterol with CREBBP, and Figure 6B shows the docking results of cerevisterol with CAT.
Table 3
| Molecule ID | Target | PDB ID | Binding energy (kcal/mol) |
|---|---|---|---|
| MOL008998 | CREBBP | 7JFM | −8.6 |
| MOL000953 | CREBBP | −7.3 | |
| MOL000358 | CREBBP | −7.8 | |
| MOL001645 | CREBBP | −4.3 | |
| MOL011169 | CREBBP | −7.7 | |
| MOL001439 | CREBBP | −4.7 | |
| MOL008999 | CREBBP | −5.9 | |
| MOL001645 | EP300 | 6V90 | −3.8 |
| MOL008999 | EP300 | −5.6 | |
| MOL000953 | EGF | 3NJP | −4.4 |
| MOL000358 | EGF | −6.7 | |
| MOL008998 | CAT | 1QQW | −9.2 |
| MOL000953 | CAT | −6.2 | |
| MOL001645 | CAT | −2.6 | |
| MOL011169 | CAT | −4.1 | |
| MOL001439 | CAT | −2.8 | |
| MOL008999 | CAT | −6.3 |
PDB, Protein Data Bank; CREBBP, CREB binding protein; EP300, E1A binding protein P300; EGF, epidermal growth factor; CAT, catalase.
Discussion
Depression is a common mood disorder, usually caused by mental stress. It is characterized by low mood and weight loss (27). Unfortunately, the current antidepressant drugs available on the market are not effective and thus, continued research into antidepressant therapy is crucial. However, it is difficult to study the antidepressant effects of Cordyceps sinensis based on traditional Chinese medicine pharmacology and instead, network pharmacology may be used to study the pharmacological mechanisms of action based on big data and computer technology (28). Network pharmacology provides a new approach to the development of traditional Chinese medicine by analyzing network properties through nodes and relationships in biological networks to elucidate drug mechanisms of action. Currently, network pharmacology is widely used in the study of TCM. For example, it can be applied to screen the active ingredients and potential targets; elucidate the complex mechanisms of drugs and prescriptions for treating diseases; and reveal the medicinal properties of herbs. However, there are still some difficulties to overcome for the further development of this discipline, such as the comprehensiveness of target information in the database needs to be improved, the protein interaction network needs to be further enhanced, and the relevant prediction results need to be experimentally verified. By combining Chinese medicine database and computer software, we not only improve the research level of traditional Chinese medicine, but also greatly promote its internationalization. The results of this present study suggested that the active ingredients in Cordyceps sinensis may exert its antidepressant effects by regulating CREB binding protein and anti-oxidative stress effects (29,30).
A total of 7 active ingredients of Cordyceps sinensis were identified using the TCMSP database, namely, arachidonic acid, linoleyl acetate, beta-sitosterol, peroxyergosterol, cerevisterol, cholesteryl palmitate, and CLR. These ingredients may have therapeutic effects in patients with depression. Arachidonic acid, linoleyl acetate, and beta-sitosterol have been associated with diseases such as Alzheimer’s disease and chronic inflammation (31-33), while CLR has been associated with brain injury (34). The antidepressant mechanisms of these components warrant further study.
The enrichment analysis revealed that the active components of Cordyceps sinensis exerted antidepressant effects mainly through the foxo signaling pathway (hsa04068), the HIF-1 signaling pathway (hsa04066), and Huntington’s disease (hsa05016). The results of GO enrichment analysis indicated that the active ingredients of Cordyceps sinensis may exert antidepressant effects through anti-oxidative stress effects and modulation of CREB binding protein (35,36). The active molecules were docked to the anti-oxidative stress-related receptor CAT, EGF (which is related to activation of MAPK activity), EP300 (which is related to activation of the TGF-β signaling pathway), and to the CREB binding protein (which is a coactivator of the cAMP response element binding protein CREB transcription factor). The receptors and ligands showed good binding, suggesting that the active ingredient of Cordyceps sinensis may act on these potential targets to achieve antidepressant effects.
The protein encoded by CREBBP has intrinsic histone acetylase activity and also acts as a scaffold that stabilizes interactions with other proteins of the transcriptional complex. This gene is involved in the transcriptional co-activation of many different transcription factors such as CREB, which plays a key role in embryonic development, growth control and homeostasis by linking chromatin remodeling to transcription factor recognition. Many target genes regulated by the cAMP signaling pathway are mediated by CREB and its phosphorylation, and ultimately regulate the transcription of genes such as brain-derived neurotrophic factor (BDNF) and tropomyosin receptor kinase B (TrkB) (37). Studies have shown that depressed patients have reduced levels of CREB phosphorylation (38), while those who are responsive to antidepressant treatment have significantly higher levels of CREB phosphorylation (39). There is evidence to suggest that oxidative stress is present in depression and that CAT protease has the ability to scavenge peroxides produced by cellular metabolism, thereby protecting cells from the toxic effects of peroxides (40). Studies have shown that very small amounts of EGF can strongly stimulate cell growth and inhibit senescence gene expression (41,42). Depression is associated with a variety of neurological disorders, such as autism (43). EP300 is a gene that expresses the p300 protein (44) which is a histone acetyltransferase that works by grafting acetyl groups on top of histones so that the gene can be expressed. Mutations in the EP300 gene may cause autism disorders (45). The active ingredients of Cordyceps sinensis may act on targets such as CREBBP, CAT, EGF, and EP300, as well as their associated pathways to achieve antidepressant effects.
Conclusions
The present study explored the antidepressant mechanisms of Cordyceps sinensis by jointly applying network pharmacology and molecular docking techniques. The results suggested that the active components of Cordyceps sinensis may exert antidepressant effects through anti-oxidative stress and modulation of the CREB binding protein (29,46). In addition, the foxo signaling pathway (hsa04068), HIF-1 signaling pathway (hsa04066), and Huntington’s disease (hsa05016) may be involved in the mechanisms of action (47-49). This study lends support to the use of computer biology to facilitate further research on Cordyceps sinensis for the treatment of patients with depression and contributes to the development of traditional Chinese medicine for future clinical applications worldwide.
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of China (Grant No. 82171863), the National Key R&D Program of China (Grant No. 2018YFC1708006), the Science and Technology Foundation Platform of Qinghai Province (Grant No. 2021-ZJ-T02), the Major Science and Technology Project of Qinghai Province (Grant No. 2021-SF-A4), the Central Asian Drug Discovery and Development Center of Chinese Academy of Sciences (Grant No. CAM201903), and Key project of Chinese Academy of Sciences (Grant No. ZDRW-ZS-2020-2).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-762/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-762/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Willner P, Scheel-Krüger J, Belzung C. The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev 2013;37:2331-71. [Crossref] [PubMed]
- Rahim T, Rashid R. Comparison of depression symptoms between primary depression and secondary-to-schizophrenia depression. Int J Psychiatry Clin Pract 2017;21:314-7. [Crossref] [PubMed]
- Ben-Efraim YJ, Wasserman D, Wasserman J, et al. Gene-environment interactions between CRHR1 variants and physical assault in suicide attempts. Genes Brain Behav 2011;10:663-72. [Crossref] [PubMed]
- Kramer IM, Simons CJ, Myin-Germeys I, et al. Evidence that genes for depression impact on the pathway from trauma to psychotic-like symptoms by occasioning emotional dysregulation. Psychol Med 2012;42:283-94. [Crossref] [PubMed]
- Vyas CM, Donneyong M, Mischoulon D, et al. Association of Race and Ethnicity With Late-Life Depression Severity, Symptom Burden, and Care. JAMA Netw Open 2020;3:e201606. [Crossref] [PubMed]
- Baniasadi N, Dehesh MM, Mohebbi E, et al. Assessing the sleep quality and depression-anxiety-stress in irritable bowel syndrome patients. Arq Gastroenterol 2017;54:163-6. [Crossref] [PubMed]
- Mikocka-Walus A, Ford AC, Drossman DA. Antidepressants in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2020;17:184-92. [Crossref] [PubMed]
- Sivolap YP, Damulin IV. Stroke and depression. Zh Nevrol Psikhiatr Im S S Korsakova 2019;119:143-7. [Crossref] [PubMed]
- Connolly KR, Thase ME. Emerging drugs for major depressive disorder. Expert Opin Emerg Drugs 2012;17:105-26. [Crossref] [PubMed]
- Liu R, Li X, Huang N, et al. Toxicity of traditional Chinese medicine herbal and mineral products. Adv Pharmacol 2020;87:301-46. [Crossref] [PubMed]
- Zhao Y, Yang A, Tu P, et al. Anti-tumor effects of the American cockroach, Periplaneta americana. Chin Med 2017;12:26. [Crossref] [PubMed]
- Zhao M, Wei D. Exploring the ligand-protein networks in traditional chinese medicine: current databases, methods and applications. Adv Exp Med Biol 2015;827:227-57. [Crossref] [PubMed]
- Long H, Qiu X, Cao L, et al. Discovery of the signal pathways and major bioactive compounds responsible for the anti-hypoxia effect of Chinese cordyceps. J Ethnopharmacol 2021;277:114215. [Crossref] [PubMed]
- Olatunji OJ, Tang J, Tola A, et al. The genus Cordyceps: An extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2018;129:293-316. [Crossref] [PubMed]
- Nishizawa K, Torii K, Kawasaki A, et al. Antidepressant-like effect of Cordyceps sinensis in the mouse tail suspension test. Biol Pharm Bull 2007;30:1758-62. [Crossref] [PubMed]
- Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 2014;6:13. [Crossref] [PubMed]
- Liu X, Ouyang S, Yu B, et al. PharmMapper server: a web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res 2010;38:W609-14. [Crossref] [PubMed]
- Wang X, Shen Y, Wang S, et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res 2017;45:W356-60. [Crossref] [PubMed]
- Wang X, Pan C, Gong J, et al. Enhancing the Enrichment of Pharmacophore-Based Target Prediction for the Polypharmacological Profiles of Drugs. J Chem Inf Model 2016;56:1175-83. [Crossref] [PubMed]
- Stelzer G, Rosen N, Plaschkes I, et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr Protoc Bioinformatics 2016;54:1.30.1-1.30.33.
- UniProt Consortium. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res 2021;49:D480-9. [Crossref] [PubMed]
- Zhou Y, Zhou B, Pache L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 2019;10:1523. [Crossref] [PubMed]
- Szklarczyk D, Gable AL, Nastou KC, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 2021;49:D605-D612. Erratum in: Nucleic Acids Res 2021;49:10800. [Crossref] [PubMed]
- Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003;13:2498-504. [Crossref] [PubMed]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 2010;31:455-61. [PubMed]
- Seeliger D, de Groot BL. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J Comput Aided Mol Des 2010;24:417-22. [Crossref] [PubMed]
- Bremner JD, Moazzami K, Wittbrodt MT, et al. Diet, Stress and Mental Health. Nutrients 2020;12:2428. [Crossref] [PubMed]
- Luo TT, Lu Y, Yan SK, et al. Network Pharmacology in Research of Chinese Medicine Formula: Methodology, Application and Prospective. Chin J Integr Med 2020;26:72-80. [Crossref] [PubMed]
- Vaváková M, Ďuračková Z, Trebatická J. Markers of Oxidative Stress and Neuroprogression in Depression Disorder. Oxid Med Cell Longev 2015;2015:898393. [Crossref] [PubMed]
- Crisafulli C, Shim DS, Andrisano C, et al. Case-control association study of 14 variants of CREB1, CREBBP and CREM on diagnosis and treatment outcome in major depressive disorder and bipolar disorder. Psychiatry Res 2012;198:39-46. [Crossref] [PubMed]
- Ghosh A, Comerota MM, Wan D, et al. An epoxide hydrolase inhibitor reduces neuroinflammation in a mouse model of Alzheimer's disease. Sci Transl Med 2020;12:eabb1206. [Crossref] [PubMed]
- Butovich IA, Lukyanova SM. Inhibition of lipoxygenases and cyclooxygenases by linoleyl hydroxamic acid: comparative in vitro studies. J Lipid Res 2008;49:1284-94. [Crossref] [PubMed]
- Ayaz M, Junaid M, Ullah F, et al. Anti-Alzheimer's Studies on β-Sitosterol Isolated from Polygonum hydropiper L. Front Pharmacol 2017;8:697. [Crossref] [PubMed]
- Kee Z, Kodji X, Brain SD. The Role of Calcitonin Gene Related Peptide (CGRP) in Neurogenic Vasodilation and Its Cardioprotective Effects. Front Physiol 2018;9:1249. [Crossref] [PubMed]
- Hritcu L, Ionita R, Postu PA, et al. Antidepressant Flavonoids and Their Relationship with Oxidative Stress. Oxid Med Cell Longev 2017;2017:5762172. [Crossref] [PubMed]
- Yan L, Xu X, He Z, et al. Antidepressant-Like Effects and Cognitive Enhancement of Coadministration of Chaihu Shugan San and Fluoxetine: Dependent on the BDNF-ERK-CREB Signaling Pathway in the Hippocampus and Frontal Cortex. Biomed Res Int 2020;2020:2794263. [Crossref] [PubMed]
- Yoo JM, Lee BD, Sok DE, et al. Neuroprotective action of N-acetyl serotonin in oxidative stress-induced apoptosis through the activation of both TrkB/CREB/BDNF pathway and Akt/Nrf2/Antioxidant enzyme in neuronal cells. Redox Biol 2017;11:592-9. [Crossref] [PubMed]
- Yang F, Wang H, Chen H, et al. RAGE Signaling pathway in hippocampus dentate gyrus involved in GLT-1 decrease induced by chronic unpredictable stress in rats. Brain Res Bull 2020;163:49-56. [Crossref] [PubMed]
- Matsushita H, Matsuzaki M, Han XJ, et al. Antidepressant-like effect of sildenafil through oxytocin-dependent cyclic AMP response element-binding protein phosphorylation. Neuroscience 2012;200:13-8. [Crossref] [PubMed]
- Lindqvist D, Dhabhar FS, James SJ, et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017;76:197-205. [Crossref] [PubMed]
- Bhora FY, Dunkin BJ, Batzri S, et al. Effect of growth factors on cell proliferation and epithelialization in human skin. J Surg Res 1995;59:236-44. [Crossref] [PubMed]
- Romana-Souza B, Silva-Xavier W, Monte-Alto-Costa A. Topical retinol attenuates stress-induced ageing signs in human skin ex vivo, throughEGFR activation viaEGF, but notERK andAP-1 activation. Exp Dermatol 2019;28:906-13. [Crossref] [PubMed]
- Galts CPC, Bettio LEB, Jewett DC, et al. Depression in neurodegenerative diseases: Common mechanisms and current treatment options. Neurosci Biobehav Rev 2019;102:56-84. [Crossref] [PubMed]
- Korzus E. Rubinstein-Taybi Syndrome and Epigenetic Alterations. Adv Exp Med Biol 2017;978:39-62. [Crossref] [PubMed]
- Zheng F, Kasper LH, Bedford DC, et al. Mutation of the CH1 Domain in the Histone Acetyltransferase CREBBP Results in Autism-Relevant Behaviors in Mice. PLoS One 2016;11:e0146366. [Crossref] [PubMed]
- Gass P, Riva MA. CREB, neurogenesis and depression. Bioessays 2007;29:957-61. [Crossref] [PubMed]
- Rana T, Behl T, Sehgal A, et al. Elucidating the Possible Role of FoxO in Depression. Neurochem Res 2021;46:2761-75. [Crossref] [PubMed]
- Li G, Zhao M, Cheng X, et al. FG-4592 Improves Depressive-Like Behaviors through HIF-1-Mediated Neurogenesis and Synapse Plasticity in Rats. Neurotherapeutics 2020;17:664-75. [Crossref] [PubMed]
- Gubert C, Renoir T, Hannan AJ. Why Woody got the blues: The neurobiology of depression in Huntington's disease. Neurobiol Dis 2020;142:104958. [Crossref] [PubMed]
(English Language Editor: J. Teoh)



