
cGAS/STING signaling in the regulation of rheumatoid synovial aggression
Introduction
Rheumatoid arthritis (RA) is a common autoimmune disorder characterized by persistent inflammation and joint destruction. Fibroblast-like synoviocytes (FLSs), which exhibit “tumorlike” properties, including increased migration and invasion, hyperproliferation, and apoptosis resistance, have a critical role in controlling synovial aggression and joint destruction (1). An increasing amount of evidence indicates that targeting FLSs might be a novel strategy for controlling joint damage in RA (2,3). However, the precise underlying mechanisms for regulating the development of the invasive phenotype of RA FLSs are still undefined.
Cytosolic DNA is a powerful activator of the innate immune system and figures prominently in autoimmune diseases. Cytosolic DNA is derived from multiple sources, including viral and bacterial pathogens or self-DNA, such as damaged mitochondrial DNA, leaked/damaged nuclear DNA, and cytosolic DNA in micronuclei (4). A previous study found elevated double-stranded DNA (dsDNA) levels in the synovial fluid of patients with RA. Interestingly, dsDNA from RA synovial fluid can induce joint inflammation in vivo (5). Our previous study also demonstrated that the accumulation of cytosolic dsDNA in RA FLSs contributes to the inflammatory response (6). Cyclic guanosine monophosphate-adenosine monophosphate (GMP‐AMP) synthase (cGAS), which serves as a key cytosolic DNA sensor, activates the stimulator of interferon genes (STING), thereby promoting type I interferon (IFN) production and causing an inflammatory response in turn (4). Our previous study showed that the accumulation of cytosolic dsDNA induces cGAS/STING activation, which promotes the proinflammatory cytokine secretion in RA FLSs, suggesting an important role for cytosolic dsDNA-mediated cGAS/STING activation in rheumatoid synovial inflammation (6). Interestingly, recent studies have indicated that cGAS/STING pathway activation is involved in modulating migration, invasion, and proliferation in some cell lines (7,8). However, it is unknown whether the cGAS/STING signaling pathway plays a role in regulating rheumatoid synovial aggression and joint destruction.
Protein kinase mammalian sterile 20-like kinase 1 (MST1), as the core component of the Hippo signaling pathway, has been implicated in the downstream control of the cGAS/STING pathway in endothelial cell migration and angiogenesis process (8). Previous studies have suggested the importance of MST1/2 in regulating cytoskeleton remodeling and integrin activation in lymphocytes (9). We were thus interested in exploring whether MST1 mediates the role of cGAS/STING signaling in modulating migration and the cytoskeleton in RA FLSs. We present the following article in accordance with the MDAR (Materials Design Analysis Reporting) reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-4533/rc).
Methods
Materials
dsDNA (ISD) and polymerase chain reaction (PCR) primers were synthesized by Sangon Company (Shanghai, China; sequences listed in Table S1). Primary antibodies against cGAS (rabbit), STING (rabbit), p-MST1 (Thr183, rabbit), MST1 (rabbit), forkhead box1 (FOXO1, rabbit), and β-actin (mouse) were purchased from Cell Signaling Technology (Danvers, MA, USA). p-FOXO1 (Ser212, rabbit) was purchased from Invitrogen (Waltham, MA, USA).
Preparation of human FLSs
Twenty active patients with RA from the First Affiliated Hospital, Sun Yat-sen University who were undergoing joint replacement or synovectomy were enrolled in our study. RA was diagnosed according to classification criteria of the 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) (10). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethics Committee of the First Affiliated Hospital, Sun Yat-sen University, China (No. [2017]049). All patients provided informed consent before participating in the study. The backgrounds of the RA patients are summarized in Table S2.
To isolate FLSs, synovial tissues were cut into small pieces and digested with collagenase for 2 hours at 37 ℃. Isolated FLSs were grown in Dulbecco’s Modified Eagle Medium/F-12 (DMEM/F12) containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin. At confluence, FLSs were routinely trypsinized, passaged, and used from passages 4 to 6.
For lentiviral transduction, cells were inoculated with lentivirus (GeneChem, Shanghai, China) at subconfluence in the presence of 10 µg/mL polybrene. After transfection for 8 hours, the media were replaced with fresh media. The target sequences of short hairpin RNA (shRNAs) are listed in Table S3.
Real-time PCR
Total RNA was extracted from FLSs and reverse-transcribed by using PrimeScript RT Master Mix (Takara, Shiga, Japan) according to the manufacturer’s protocol. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis was performed using a Light Cycler 480 Real-Time PCR Detection System (Roche, Basel, Switzerland) and SYBR Premix Ex Taq (Takara) according to the manufacturers’ instructions. The relative messenger RNA (mRNA) expression levels were normalized to the GAPDH (ΔCt = Ct target − Ct GAPDH) and compared with a calibrator using the ΔΔCt method (ΔΔCt = ΔCt sample − ΔCt calibrator). Table S1 shows the primer sequences. All experiments were performed at least 3 times.
Gene silencing
cGAS small interfering RNA (siRNA), STING siRNA, MST1 siRNA, FOXO1 siRNA, and yes-associated protein (YAP) siRNA were purchased from Ribobio (Guangzhou, China). The target sequences of the siRNAs are listed in Table S4. RA FLSs that reached 60–70% confluence were transfected with a mixture of 100 nM siRNA and 10 mg/mL Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, USA) for 6 hours, according to the manufacturer’s protocols. Experiments were performed 48 hours after transfection. At the end of the culture, the transfection efficiency was confirmed using western blot and qRT-PCR.
In vitro migration and invasion assay of FLS
Cell migration ability was assessed with the Boyden chamber method using a filter with an 8.0-µm pore size and a 6.5 mm diameter (Corning Life Sciences, Tewksbury MA, USA). FLSs (6×104 cells/mL) were suspended in serum-free DMEM in the upper wells, while DMEM containing 10% FBS as a chemoattractant was placed in the lower wells. The chamber was incubated at 37 ℃ for 6 hours. After incubation, the remaining cells on the upper surface of the filter were scraped using a cotton bud. The migrated cells in the filter were fixed in methanol for 15 minutes, stained with 0.1% crystal violet for 15 minutes, and counted using an optical microscope. The stained cells were quantified as the mean number of cells per 10 random fields for each assay. Transwell chambers precoated with Matrigel matrix (BD Biosciences, Franklin Lakes, NJ, USA) were used for invasion assay.
F-actin staining
RA FLSs were cultured on 18 mm coverslips. The cells were fixed in 4% paraformaldehyde for 15 minutes and permeabilized by 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 5 minutes at room temperature and then blocked in a blocking buffer for 1 hour. Cells were incubated overnight with phalloidin at 4 ℃ to detect F-actin and then stained using 4',6-diamidino-2-phenylindole (DAPI; BBI, Shanghai, China) to detect nuclei. The coverslips were mounted using fluoroshield histology mounting medium (Sigma-Aldrich, St. Louis, MO, USA) and examined using a fluorescence microscope (DMI8, Leica, Wetzlar, Germany).
Immunofluorescence
FLSs were briefly washed in PBS, fixed in 4% paraformaldehyde in PBS for 15 minutes, permeabilized with 0.1% Triton X-100 in PBS for 15 minutes, and blocked in a blocking buffer for 1 hour at room temperature. After incubation with diluted primary antibodies overnight at 4 ℃ and fluorochrome-conjugated secondary antibody (Invitrogen) for 1 hour at room temperature, the cell nuclei were stained using DAPI (BBI) and imaged using fluoroshield histology mounting medium (Sigma-Aldrich).
Western blotting
Protein concentrations were quantified using the bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific). The cell lysates prepared in sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE) Sample Loading Buffer (Beyotime, Shanghai, China) were separated by 10–12% SDS-PAGE and transferred onto a 0.2-µm polyvinylidene fluoride (PVDF) membrane (Millipore, Burlington, MA, USA). The PVDF membranes were incubated with indicated primary antibodies diluted 1:1,000 for cGAS, STING, p-MST1, MST1, p-FOXO1, FOXO1, LATS1, and YAP.
Intracellular ROS detection
2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA) (Beyotime) was used to detect intracellular ROS levels of FLSs. FLSs were incubated with 10 µM of DCFH-DA in serum-free DMEM/F12 at 37 ℃ for 20 minutes. Cells were washed 3 times to remove excessive DCFH-DA and subjected to flow cytometry (CytoFLEX S, Beckman Coulter, Brea, CA, USA), with an emission wavelength of 525 nm and an excitation wavelength of 488 nm.
Assessment of the in vivo invasion of RA FLSs into human cartilage implants
RA FLSs (4×105) were suspended in 100 µL of sterile saline and absorbed by a gelatin sponge (80 mm3). Human cartilage obtained from non-arthritic trauma patients undergoing knee surgery was cut into pieces and inserted into the sponge. For implantation, each sponge containing cartilage and RA FLSs was placed under the skin on the left flanks of 4-week-old SCID mice (Sun Yat-sen University Laboratory Animal Center, Guangzhou, China). All mice were housed under specific pathogen-free (SPF) conditions and allowed free access to water and food. After 50 days of implantation, the implants were taken out from the mice, and the mice were killed. Each explant specimen was stained with hematoxylin and eosin (HE). FLS invasion into cartilage was graded as previously described (11). The invasiveness level was scored as follows: 0 = no or minimal invasion, 1 = visible invasion (2-cell depth), 2 = invasion (5-cell depth), and 3 = deep invasion (more than 10-cell depth). Each specimen was evaluated in a blinded manner by 2 experienced investigators. All procedures involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Sun Yat-sen University (No. SYSU-IACUC-2021-000028) and conducted according to the guidelines of the Chinese Ethics Committee for the care and use of animal research.
Statistical analysis
All statistical analyses of the data were performed using SPSS 13.0 (IBM Corp., Armonk, NY, USA) and analyzed in a blinded manner. Data are expressed as the means ± standard error of the mean (SEM). Presented values were derived from at least 3 independent experiments. A 2-tailed Student’s t-test was used to analyze the differences between 2 groups, and 1-way analysis of variance (ANOVA) analysis of variance with Bonferroni’s post hoc comparisons was used to analyze 3 or more groups in normally distributed data. We used nonparametric tests (Mann-Whitney rank-sum test for 2 groups or the Kruskal-Wallis 1-way analysis among 3 groups for continuous variables) to compare the differences between different groups in abnormal distribution data. P values less than 0.05 were considered significant.
Results
The cGAS/STING pathway regulated cytosolic dsDNA-induced migration and invasion of RA FLSs
The abnormal migration and invasion of FLSs play a key role in controlling rheumatoid synovial aggression and joint destruction. Our previous study found an increased accumulation of cytosolic dsDNA and expression of cGAS and STING in FLSs and synovial tissues from patients with RA. In addition, a positive correlation between the cGAS immunoreactive score (IRS) and synovitis score was observed (6). Herein, we evaluated the role of cytosolic dsDNA-mediated cGAS/STING signaling in regulating the migration and invasion of RA FLSs. Specific siRNAs were used to inhibit cGAS or STING expression. As shown in Figure 1A, transfection with all 3 siRNA oligonucleotides decreased cGAS or STING expression; however, the inhibitory effect of siRNA-1 and siRNA-2 on cGAS or siRNA-2 and siRNA-3 on STING was more prominent. Thus, siRNA-1 and siRNA-2 for cGAS or siRNA-2 and siRNA-3 for STING were used for further experiments. We transfected ISD dsDNA (45 bp dsDNA) into the RA FLSs to activate the cGAS/STING pathway. As shown in Figure 1B, treatment with ISD dsDNA promoted the migration of RA FLSs; however, this promotion was mitigated in cGAS or STING siRNA-transfected RA FLSs. Since the ability to invade cartilage and bone is a critical pathogenic behavior of RA FLSs, we examined the effect of cGAS or STING suppression on modulating the invasive behavior of RA FLSs using Matrigel-coated Transwell membranes. We determined that ISD dsDNA stimulation increased the invasion of RA FLSs, whereas the transfection with cGAS or STING siRNA diminished ISD dsDNA-induced invasion of RA FLSs (Figure 1C).
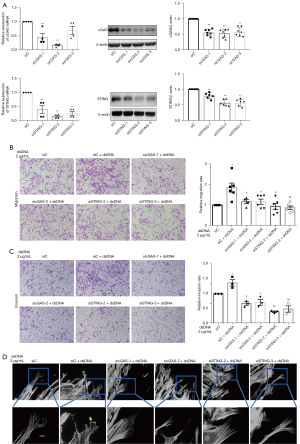
Cell migration requires the dynamic reorganization of the actin cytoskeleton. We used fluorescent phalloidin staining to observe polymerized actin in ISD-induced migrating cells after wounding in cGAS- or STING-inhibited RA FLSs. As shown in Figure 1D, RA FLSs with ISD stimulation displayed lamellipodia, while RA FLSs with cGAS or STING knockdown exhibited reduced formation of lamellipodia. Our data indicate a role for the cGAS/STING pathway in the formation of membrane protrusions in migrating cells.
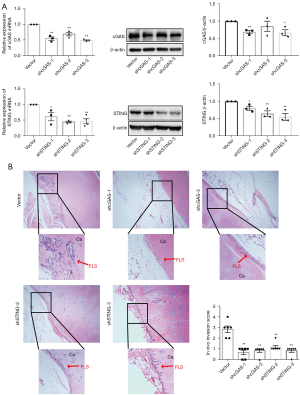
Finally, we evaluated the effect of cGAS or STING knockdown on the in vivo invasion of RA FLSs into cartilage using the SCID mouse coimplantation model. Specific shRNAs were used to inhibit cGAS or STING expression. As shown in Figure 2A, transfection with all 3 lentiviral shRNAs decreased cGAS or STING expression; however, the inhibitory effect of shRNA-1 and shRNA-3 on cGAS or shRNA-2 and shRNA-3 on STING was more prominent. Thus, shRNA-1 and shRNA-3 for cGAS or shRNA-2 and shRNA-3 for STING were used for subsequent experiments. RA FLSs carrying cGAS or STING shRNA or empty vector were coimplanted side by side into the left or right flanks of SCID mice. RA FLSs transfected with cGAS or STING shRNA exhibited a significantly decreased of invasion into cartilage as compared to the cells transfected with empty vector (Figure 2B). Collectively, our findings suggested that the cGAS/STING pathway is important for regulating the migration and invasion of RA FLSs.
MST1 mediated the cGAS/STING regulation of RA FLS function
A previous study showed that the cGAS/STING pathway is involved in cell migration through regulating the activation of MST1 (8). Therefore, we investigated whether MST1 mediates the role of the cGAS/STING pathway in the cytosolic dsDNA-induced migration and invasion of RA FLSs. As shown in Figure 3A, ISD dsDNA transfection promoted MST1 phosphorylation with a peak at 2 hours; however, this promotion was mitigated by cGAS or STING knockdown (Figure 3B).
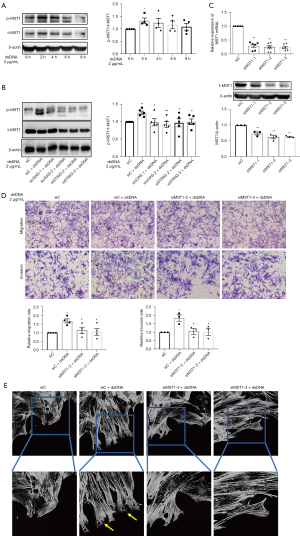
We further determined the role of MST1 in RA FLS migration and invasion by using 2 specific MST1 siRNAs to inhibit MST1 expression (Figure 3C). We found that MST1 knockdown inhibited the enhanced ISD dsDNA-induced migration and invasion of RA FLSs (Figure 3D). Consistent with our findings regarding migration and invasion, RA FLSs with MST1 knockdown exhibited decreased formation of lamellipodia (Figure 3E). These data suggested that MST1 acts downstream of the cGAS/STING pathway in the cytosolic dsDNA-induced migration and invasion of RA FLSs.
MST1 regulated the migration and invasion of RA FLSs independent of the canonical Hippo pathway
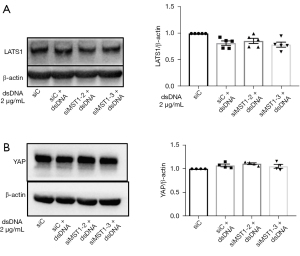
To determine whether canonical large tumor suppressor kinase 1 (LATS1) and YAP are the downstream regulators of MST1 in RA FLSs, we assessed the effect of MST1 inhibition on LATS1 and YAP protein expression. We discovered that MST1 knockdown did not influence the protein expression of LATS1 or YAP (Figure 4). These findings suggested that MST1 does not use the canonical Hippo signaling pathway for directing migration of RA FLSs.
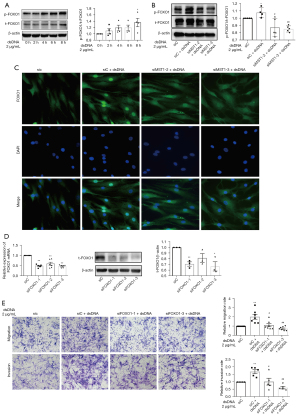
FOXO1 mediated MST1 regulation for the migration and invasion of RA FLSs
In canonical signaling, MST1/2 activation leads to phosphorylation of LATS1/2, and subsequently regulates the Hippo pathway effector Yorkie (YAP/TAZ) (12). Thus, to explore the underlying mechanisms by which MST1 modulates the migration and invasion of RA FLSs, we clarified the effect of MST1 knockdown on the expression of LATS1 and YAP. MST1 knockdown did not influence the protein expression of LATS1 or YAP (Figure 4), suggesting that MST1 does not use the canonical Hippo pathway for the migration of RA FLSs.
Therefore, we focused on the alternative downstream effector of MST1 beyond the canonical Hippo pathway. According to the findings on other cell types in previous reports, FOXO1 may be a downstream cellular substrate of MST1. As shown in Figure 5A, phosphorylation of FOXO1 reached a peak at 8 hours in response to ISD stimulation. Transfection with MST1 siRNA decreased the phosphorylation of FOXO1 (Figure 5B). Moreover, we observed that ISD dsDNA stimulation resulted in the nuclear translocation of FOXO1 in RA FLSs; however, this translocation was reversed in MST1 siRNAs-transfected cells (Figure 5C). To clarify the role of FOXO1 in RA FLS migration and invasion, we used siRNA to knockdown FOXO1 (Figure 5D). FOXO1 siRNA-transfected RA FLSs exhibited reduced migration and invasion (Figure 5E). Our findings implied that FOXO1 may be a downstream substrate of MST1 for regulating the cytosolic dsDNA-induced migration and invasion of RA FLSs.
cGAS/STING regulated the level of reactive oxygen species production in RA FLSs
We next sought to gain mechanistic insights into how cGAS/STING modulates MST1 expression in RA FLSs. It has been reported that MST1 is sensitive to oxidative stress (13); therefore, we investigated whether reactive oxygen species (ROS) production is involved in the role of cGAS/STING in controlling MST1. 2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA) was used to measure the ROS production. We first evaluated the effect of ISD dsDNA stimulation on ROS production and found that the ROS levels peaked 8 hours after ISD dsDNA stimulation (Figure 6A). The major sources of ROS include the NADPH oxidases, xanthine oxidase, and mitochondria; therefore, we used a series of pharmacological inhibitors to determine the sources of ROS induced by ISD dsDNA. Our results showed that rotenone (mitochondrial complex I inhibitor) or antimycin A (mitochondrial complex III inhibitor) abrogated ISD dsDNA-induced ROS production for 8 hours, while apocynin (NADPH oxidase inhibitor), AEBSF (NADPH oxidase inhibitor), carboxine (xanthine oxidase inhibitor), or oxypurinol (xanthine oxidase inhibitor) treatment did not affect ROS production, indicating that the mitochondrial ROS are the major source induced by ISD dsDNA (Figure 6B).
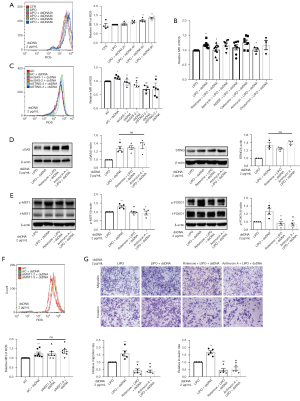
Next, we evaluated the effect of cGAS or STING inhibition on ROS production. Our results showed that cGAS or STING knockdown decreased the ROS levels induced by ISD dsDNA (Figure 6C), while treatment with rotenone or antimycin A did not influence the cGAS or STING expression (Figure 6D), implying that ROS induction occurs downstream of the cGAS/STING pathway. Finally, we investigated the role of ROS in regulating MST1 and FOXO1 activity. As shown in Figure 6E, treatment with rotenone or antimycin A diminished the phosphorylation of MST1 and FOXO1 in RA FLSs; however, MST1 knockdown did not affect ISD dsDNA-induced ROS levels (Figure 6F). These data indicated that ROS operate upstream of the MST1-FOXO1 pathway.
As a previous study reported an increased amount of mitochondrial ROS in RA FLSs (14), we aimed to further determine whether mitochondrial ROS affects the migration and invasion of RA FLSs. Our results showed that treatment with rotenone or antimycin A reduced the ISD dsDNA-induced migration and invasion of RA FLSs (Figure 6G). These findings suggested that mitochondrial ROS play an important role in regulating the migration and invasion of RA FLSs induced by ISD dsDNA.
Discussion
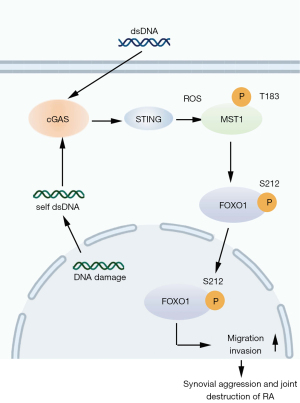
We found that transfection with synthetic dsDNA promotes the migration and invasion of RA FLSs, while cGAS or STING knockdown decreases dsDNA-induced migration and invasion. Furthermore, our results showed that cGAS/STING activation leads to increased mitochondrial ROS levels that promote MST1 phosphorylation and subsequent phosphorylation and nuclear translocation of FOXO1. Furthermore, cGAS/STING activation may contribute to the aggressive behavior of RA FLSs by targeting MST1-mediated FOXO1 (Figure 7).
Recent studies have indicated that the cGAS/STING pathway participates in regulating the migration of some cell lines and tumor metastasis. For instance, cytosolic mitochondrial DNA (mtDNA) can activate cytosolic DNA sensor cGAS/STING signaling to regulate endothelial cell migration and angiogenesis processes (8). Furthermore, STING knockdown was found to enhance cervical cancer cell migration (15), while cGAS/STING signal activation was shown to promote the maturation and migration of dendritic cells (16). In our study, we demonstrated that cGAS or STING inhibition also decreased the migration and invasion of cytosolic dsDNA-induced RA FLSs. Moreover, cGAS or STING knockdown blocked the invasion of RA FLSs into human cartilage implants transferred under the skin of SCID mice. A growing body of evidence indicates that the high invasive ability of FLSs is critical for joint destruction in RA; therefore, our findings suggest that the cGAS/STING pathway has an important role in controlling rheumatoid synovial aggression and joint destruction.
Hippo kinase MST1 has emerged as a critical downstream target of the cGAS/STING pathway in the process of endothelial cell migration (8). To explore how the cGAS/STING pathway regulates RA FLSs functions, we evaluated the role of cGAS/STING in modulating MST1 activation in RA FLSs. We determined that cGAS or STING knockdown suppressed dsDNA-induced MST1 phosphorylation and that MST1 inhibition reduced the migration and invasion of RA FLSs, suggesting that MST1 mediates the role of cGAS/STING in controlling RA FLS migration. Consistent with our findings, it has been reported that MST1 promotes the malignant proliferation and metastasis of tumor cells (17) and that MST1 knockdown inhibits the proliferation and migration of T cells from psoriasis patients, indicating that MST1 may be a target for the treatment of psoriasis (18). However, there are also reports that MST1 overexpression inhibits the proliferation and migration of breast cancer cells (19), suggesting that MST1 might possess a cell type-specific role in regulating cellular functions.
We further explored how MST1 controls RA FLS functions. It is well known that the Hippo signaling pathway figures prominently in development, tissue homeostasis, and immune response. Dysregulation of Hippo signaling results in abnormal cell proliferation and neoplasia (20). In mammalian cells, Hippo signaling can be initiated by a series of upstream stimuli. In canonical signaling, activation of MST1/2, a core component of Hippo, results in subsequent phosphorylation of LATS1/2, and negatively modulates the Hippo pathway effector Yorkie (YAP/TAZ) (12). Therefore, we evaluated whether MST1 regulates the phosphorylation of LATS1 and YAP. We found that MST1 knockdown did not affect the phosphorylation of LATS1/2 or YAP in dsDNA-induced RA FLSs. This indicates that the LATS1/YAP pathway does not mediate the role of MST1 in regulating RA FLS functions. Similar to our results, netrin-1-induced YAP protein stability is not dependent on the Hippo kinase MST1/2 in various liver and glioblastoma cancer cells (21). Therefore, we speculated that MST1 controls RA FLSs functions through alternative pathways. Recent evidence shows that MST1 regulates various cellular processes by activating the noncanonical Hippo pathways or alternative pathways and not the “canonical” Hippo pathway (22). Interestingly, recent studies show that MST1 phosphorylates the transcription factor FOXO1, resulting in oxidative stress-induced neuronal cell death (23,24). The endothelial MST1-FOXO1 cascade is critical for the directional migration of endothelial tip cells (24). Moreover, phosphorylation of FOXO1 has been observed in RA FLSs (25). This prompted us to ascertain whether FOXO1 mediates the role of MST1 in RA FLS functions. As expected, we found that MST1 knockdown decreased the dsDNA-induced phosphorylation of FOXO1 and that cGAS or STING knockdown also inhibited the dsDNA-induced phosphorylation of FOXO1. Furthermore, FOXO1 knockdown decreased the migration and invasion of RA FLSs. Collectively, these findings suggest that MST1-mediated cGAS/STING controls RA FLS migration and invasion via FOXO1 and not via the “canonical” LATS1-YAP pathway.
It has been reported that ROS-mediated regulation of cell migration and tumor metastasis is dependent on the noncanonical Hippo MST1/MST2-FoxO3a-ΔNp63α pathway (26). ROS produced in the mitochondria can activate MST1 in endothelial cells, promoting the nuclear import of FOXO1 and augmenting its transcriptional regulation of polarity and migration-associated genes (27), which suggests that MST1 is sensitive to oxidative stress. In our study, we found that cGAS or STING knockdown reduced ROS production and that a mitochondrial ROS inhibitor diminished the phosphorylation of MST1 in ISD dsDNA-stimulated RA FLSs. Consistent with our findings, a previous study showed that oxidative stress stimulation increased MST1 expression and promoted mitochondrial dysfunction in RA FLSs (13). We also observed that a mitochondrial ROS inhibitor reduced ISD dsDNA-induced migration and invasion of RA FLSs. Taken together, our findings suggest that the cGAS/STING activation may elicit mitochondrial ROS production, leading to MST1 phosphorylation in RA FLSs.
In summary, we demonstrated that cGAS/STING signaling plays an important role in controlling rheumatoid synovial aggression via the mitochondrial ROS–mediated MST1-FOXO1 pathway. This suggests that targeting cGAS/STING signaling might be a potential therapeutic strategy for RA.
Acknowledgments
The authors would like to thank Prof. Aishan He (Sun Yat-sen University, China) for providing a portion of the clinical samples.
Funding: This work was supported by grants from the National Natural Science Foundation of China (grants Nos. 81871275, 82071831, 81671591, 82001742, U1401222, and 81501389), the Guangzhou Science and Technology Project (Nos. 201803010042, and 202102020238), and the Guangdong Basic and Applied Basic Research Foundation (Nos. 2020A1515010221, 2021A1515010535).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4533/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4533/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-21-4533/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-4533/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- You S, Koh JH, Leng L, et al. The Tumor-Like Phenotype of Rheumatoid Synovium: Molecular Profiling and Prospects for Precision Medicine. Arthritis Rheumatol 2018;70:637-52. [Crossref] [PubMed]
- Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol 2013;9:24-33. [Crossref] [PubMed]
- Svensson MND, Zoccheddu M, Yang S, et al. Synoviocyte-targeted therapy synergizes with TNF inhibition in arthritis reversal. Sci Adv 2020;6:eaba4353. [Crossref] [PubMed]
- Yu L, Liu P. Cytosolic DNA sensing by cGAS: regulation, function, and human diseases. Signal Transduct Target Ther 2021;6:170. [Crossref] [PubMed]
- Dong C, Liu Y, Sun C, et al. Identification of Specific Joint-Inflammatogenic Cell-Free DNA Molecules From Synovial Fluids of Patients With Rheumatoid Arthritis. Front Immunol 2020;11:662. [Crossref] [PubMed]
- Wang J, Li R, Lin H, et al. Accumulation of cytosolic dsDNA contributes to fibroblast-like synoviocytes-mediated rheumatoid arthritis synovial inflammation. Int Immunopharmacol 2019;76:105791. [Crossref] [PubMed]
- Guo Q, Chen X, Chen J, et al. STING promotes senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the NF-κB signaling pathway. Cell Death Dis 2021;12:13. [Crossref] [PubMed]
- Yuan L, Mao Y, Luo W, et al. Palmitic acid dysregulates the Hippo-YAP pathway and inhibits angiogenesis by inducing mitochondrial damage and activating the cytosolic DNA sensor cGAS-STING-IRF3 signaling mechanism. J Biol Chem 2017;292:15002-15. [Crossref] [PubMed]
- Ueda Y, Kondo N, Kinashi T. MST1/2 Balance Immune Activation and Tolerance by Orchestrating Adhesion, Transcription, and Organelle Dynamics in Lymphocytes. Front Immunol 2020;11:733. [Crossref] [PubMed]
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569-81. [Crossref] [PubMed]
- Müller-Ladner U, Kriegsmann J, Franklin BN, et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol 1996;149:1607-15. [PubMed]
- Taha Z, Janse van Rensburg HJ, Yang X. The Hippo Pathway: Immunity and Cancer. Cancers (Basel) 2018;10:94. [Crossref] [PubMed]
- Wang Y, Yang Q, Shen S, et al. Mst1 promotes mitochondrial dysfunction and apoptosis in oxidative stress-induced rheumatoid arthritis synoviocytes. Aging (Albany NY) 2020;12:16211-23. [Crossref] [PubMed]
- Fan XX, Xu MZ, Leung EL, et al. ROS-Responsive Berberine Polymeric Micelles Effectively Suppressed the Inflammation of Rheumatoid Arthritis by Targeting Mitochondria. Nanomicro Lett 2020;12:76. [Crossref] [PubMed]
- Shi F, Su J, Wang J, et al. Activation of STING inhibits cervical cancer tumor growth through enhancing the anti-tumor immune response. Mol Cell Biochem 2021;476:1015-24. [Crossref] [PubMed]
- Li A, Yi M, Qin S, et al. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J Hematol Oncol 2019;12:35. [Crossref] [PubMed]
- Hong AW, Meng Z, Guan KL. The Hippo pathway in intestinal regeneration and disease. Nat Rev Gastroenterol Hepatol 2016;13:324-37. [Crossref] [PubMed]
- Tang H, Guo Z, Tang X, et al. MST1 modulates Th17 activation in psoriasis via regulating TLR4-NF-κB pathway. Hum Cell 2021;34:28-36. [Crossref] [PubMed]
- Jin X, Zhu L, Xiao S, et al. MST1 inhibits the progression of breast cancer by regulating the Hippo signaling pathway and may serve as a prognostic biomarker. Mol Med Rep 2021;23:383. [Crossref] [PubMed]
- Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev 2016;30:1-17. [Crossref] [PubMed]
- Qi Q, Li DY, Luo HR, et al. Netrin-1 exerts oncogenic activities through enhancing Yes-associated protein stability. Proc Natl Acad Sci U S A 2015;112:7255-60. [Crossref] [PubMed]
- Chen L. Non-canonical Hippo signaling regulates immune responses. Adv Immunol 2019;144:87-119. [Crossref] [PubMed]
- Lehtinen MK, Yuan Z, Boag PR, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 2006;125:987-1001. [Crossref] [PubMed]
- Yuan Z, Lehtinen MK, Merlo P, et al. Regulation of neuronal cell death by MST1-FOXO1 signaling. J Biol Chem 2009;284:11285-92. [Crossref] [PubMed]
- Ludikhuize J, de Launay D, Groot D, et al. Inhibition of forkhead box class O family member transcription factors in rheumatoid synovial tissue. Arthritis Rheum 2007;56:2180-91. [Crossref] [PubMed]
- Wang Y, Li J, Gao Y, et al. Hippo kinases regulate cell junctions to inhibit tumor metastasis in response to oxidative stress. Redox Biol 2019;26:101233. [Crossref] [PubMed]
- Kim YH, Choi J, Yang MJ, et al. A MST1-FOXO1 cascade establishes endothelial tip cell polarity and facilitates sprouting angiogenesis. Nat Commun 2019;10:838. [Crossref] [PubMed]
(English Language Editors: C. Mullens and J. Gray)


