
Sofosbuvir and ledipasvir decreased nephrotic syndrome caused by IgA nephropathy with a membranoproliferative pattern of injury in hepatitis C virus-induced cirrhosis: a case report
Introduction
Membranoproliferative glomerulonephritis (MPGN) and mixed cryoglobulinemia are known to be associated with hepatitis C virus (HCV) infection. One cohort study showed a 2- and 17-fold higher risk of MPGN and cryoglobulinemia, respectively, in chronic HCV patients compared with matched non-HCV patients (1). Another survey found a greater proportion of MPGN than membranous nephropathy (MN) in HCV-infected patients (2). Other pathological types such as focal segmental glomerulosclerosis (FSGS) and immunoglobulin (Ig) A nephropathy (IgAN) have been occasionally reported (3). Treating HCV infection effectively is shown to reduce the risk of chronic kidney disease (CKD) (1).
In liver cirrhosis, IgAN is the most common type of kidney pathology (4). IgA deposits in the kidney are hypothesized to result from decreased clearance of IgA immune complex and collateral shunt formation due to portal hypertension (5). Case reports suggest that treatment of liver cirrhosis or portal hypertension can decrease kidney pathological manifestation (6). However, due to the higher complication risk for kidney biopsy in liver cirrhosis, IgAN in HCV cirrhosis is understudied. It is not known whether eradication of HCV by direct-acting antiviral (DAA) drugs can lead to remission of renal manifestations and improve the long-term prognosis.
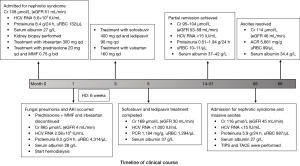
We report the case of a patient with HCV cirrhosis that presented with nephrotic syndrome (NS), which was caused by IgAN with a membranoproliferative pattern of injury. Partial remission of NS was achieved after HCV eradication by treatment with sofosbuvir and ledipasvir. The first NS relapse occurred 5 years later with deterioration of portal hypertension and suspected hepatic carcinoma despite an HCV sustained virologic response (SVR) (Figures 1,2). We present the following case in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-21-5289/rc) (7).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A 52-year-old Chinese woman presented with NS for 3 months and was admitted to our department in December 2014. The patient’s medical history included HCV cirrhosis for 10 years without antiviral treatment, type 2 diabetes mellitus for 10 years treated by insulin, and hypertension for 3 years well controlled by 160 mg valsartan. She had undergone splenectomy and cholecystectomy in 2005 due to complications of liver cirrhosis.
On physical examination, she had moderate symmetrical edema in her lower limbs and eyelid, and mild hypertension (blood pressure, 143/90 mmHg). Urinalysis and blood biochemistry tests showed the following results: 24-hour proteinuria, 8.4 g; urinary red blood cell (uRBC), 132/µL (refence range, 0–26/µL); serum albumin, 27 g/L; creatinine, 108 µmol/L; estimated glomerular filtration rate (eGFR), 51 mL/minute (all eGFR values in this paper were estimated using the 2009 CKD-Epidemiology Collaboration creatinine equation); hemoglobin, 109 g/L; HCV RNA quantification, 5.6×103 IU/mL; and HbA1c, 5.1% (Table 1). Additionally, cryoglobulin, antinuclear antibody (ANA), extractable nuclear antigen (ENA), and anti-neutrophil cytoplasmic antibody (ANCA) test results were all negative. The humoral immune assay revealed the following results: serum complement 3 (C3), 0.43 g/L (refence range, 0.8–1.6 g/L); C4, 0.13 g/L (0.1–0.4 g/L); IgG, 8.28 g/L (8–16 g/L); IgA, 5.1 g/L (0.7–3.3 g/L); and C-reactive protein (CRP), 3.9 mg/L (0–6 g/L). The HCV genotype was 1b. Ultrasound examination indicated normal-sized kidneys and confirmed the diagnosis of liver cirrhosis, while the right portal vein branches could not be visualized. Gastroscopy revealed esophageal and gastric varices, which suggested portal hypertension.
Table 1
| Date (year-month) | 2014-12 | 2015-01 | 2015-02 | 2015-03 | 2015-04 | 2015-05 | 2015-08 | 2015-11 | 2016-02 | 2016-12 | 2017-04 | 2017-11 | 2018-03 | 2018-06 | 2019-03 | 2020-05 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month | 0 | 1 | 2 | 3 | 4 | 5 | 8 | 11 | 14 | 24 | 28 | 35 | 39 | 42 | 51 | 65 |
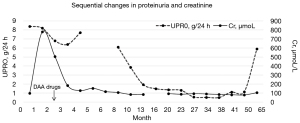
| UPRO (g/24 hours) | 8.4 | 8.2 | 6.79 | 6.4 | 7.7 | ND | 6.1 | 3.85 | 1.94 | 1.3 | 0.56 | 0.52 | 0.51 | 1.11 | 1.14 | 5.9 |
| URBC (n/μL) | 132 | 4,314 | ND | ND | ND | 1,294 | 148 | 33 | ND | 10 | 11 | 9 | ND | ND | 0 | 887 |
| Cr (μmol/L) | 108 | 865 | 562 | 205 | 142 | 169 | 118 | 94 | 95 | 97 | 104 | 100 | 94 | 90 | 88 | 116 |
| eGFR (mL/minute) | 51 | 4 | 7 | 23 | 36 | 30 | 46 | 60 | 59 | 58 | 53 | 56 | 60 | 63 | 65 | 45 |
| ALB (g/L) | 27 | 28 | 30.8 | 35.4 | 27.9 | 37 | 37.7 | 33.5 | 37.3 | 41.6 | 41.8 | 42 | 40 | 38.3 | 39.9 | 27 |
| PT-INR | 1.14 | 1.17 | 1.35 | 1.24 | 1.27 | 0.92 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.98 |
| TBIL (μmol/L) | 21 | 19 | 30.9 | ND | ND | ND | 12.1 | 14.8 | 18 | 13.6 | 22.7 | 14.2 | 13.9 | 15.9 | 12.8 | 15 |
| HCV-RNA (IU/mL) | 5.6×103 | 2.58×103 | ND | <103 | ND | <103 | ND | <15 | ND | ND | <15 | ND | ND | <15 | <5×102 | <15 |
| Hb (g/L) | 109 | 84 | 81 | 94 | 100 | 119 | 119 | ND | 127 | 136 | 140 | 140 | ND | ND | 148 | 158 |
| HbA1c (%) | 5.1 | ND | ND | ND | ND | ND | ND | ND | ND | 7.3 | 6.2 | 6.2 | 6.5 | 6.2 | 6.8 | 5.4 |
| IgA (g/L) | 5.1 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 3.5 |
Month, number of months since admission; UPRO, 24-hour quantification of urine protein; ND, not done; uRBC, red blood cell count in urine (reference range, 0–26/μL); eGFR, estimated glomerular filtration rate calculated using CKD-EPI 2009 equation; ALB, albumin; PT-INR, prothrombin time-international normalized ratio; TBIL, total bilirubin (reference range, 4–23.9 μmol/L); HCV-RNA, quantification of HCV ribose nucleic acid; Hb, hemoglobin; IgA, immunoglobulin A (reference range 0.7–3.3 g/L).
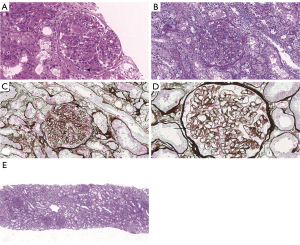
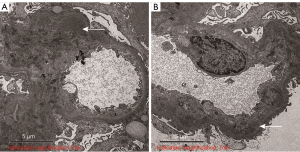
Ultrasound-guided percutaneous renal biopsy was performed by an experienced specialist using a 16-gauge needle (BARD Max-CORE MC1616, 1625 west 3rd street, Tempe, Arizona 85281, USA). Renal histology revealed 11 glomeruli in the specimen including one with segmental fibro cellular crescents and none with global or segmental sclerosis. Some glomeruli were slightly enlarged due to mesangial expansion. Significant endothelial hyperplasia and mononuclear and polymorphonuclear leukocyte infiltration were noted. Splitting of the glomerular basement membrane with subendothelial deposits and mesangial deposits was found using periodic acid-silver methenamine-Masson staining. The interstitium showed mild tubular atrophy with insignificant fibrosis. Positive IgA and C3 deposits with an intensity of 2+ were found in the mesangium using an immunofluorescent analysis with no IgG, IgM, or C1q deposition. No HCV antigen was detected using immunohistochemistry. The patient’s pathological diagnosis was IgAN with a membranoproliferative pattern of injury, which was made by a renal pathologist with more than 10 years of experience (Figure 3). Electron microscopy revealed vacuolar degeneration of endothelial and tubular epithelial cells, effacement of podocyte foot processes and electron-dense deposits in the mesangial and subendothelial region. These results thus confirmed the diagnosis (Figure 4).
Antiviral therapy was not initially prescribed, because DAA medication was not available in China at that time. The patient did not respond to furosemide, tolvaptan, irbesartan, or intravenous albumin infusion and presented with acute kidney injury (AKI), ascites, and oliguria during hospitalization. Thus, prednisolone 20 mg once daily and mycophenolate mofetil (MMF) 0.75 g twice daily were administered as an immunosuppressive regimen. The regimen was discontinued after 30 days because of new-onset fungal pneumonia when NS did not resolve and the patient was on hemodialysis due to AKI. The laboratory tests showed the following results: 24-hour proteinuria, 6.8 g; uRBC, 4,314 /µL; serum albumin, 30 g/L; serum creatinine 606 µmol/L; eGFR, 6 mL/minute; and hemoglobin, 103 g/L. Sofosbuvir 400 mg plus ledipasvir 90 mg (brand name: Harvoni) was obtained and prescribed once a day for 12 weeks (Figure 1). The patient also received antifungal medication and underwent hemodialysis. The patient’s symptoms resolved and dialysis was withdrawn. The duration of hemodialysis was 6 weeks. Valsartan 160 mg once daily was administered when the creatinine declined to baseline level. The HCV viral load dropped below the detection limit in May 2015, and a SVR was achieved in November 2015. Partial remission of IgAN was achieved in February 2016 (24-hour proteinuria, 1.9 g; serum albumin 37.3 g/L; serum creatinine 95 µmol/L; eGFR, 59 mL/minute; Table 1, Figure 2).
Five years later (May 2020), the patient was readmitted to our department for 2-month abdominal distension. Upon examination, we found that the patient had massive ascites with mild lower limb edema. Laboratory tests revealed the following results: 24-hour proteinuria, 5.9 g; uRBC, 887/µL; serum albumin 27 g/L; creatinine 116 μmol/L; eGFR, 45 mL/minute; hemoglobin, 158 g/L; HbA1c, 5.4%; alpha-fetoprotein (AFP), 2.89 ng/mL; C3, 1.45 g/L; C4, 0.34 g/L; IgG, 12.46 g/L; IgA, 3.47 g/L; and an HCV SVR (Table 1). Fundus photography revealed no diabetic retinopathy. Progression of esophageal and gastric varices was identified using gastroscopy. Neither portal nor hepatic veins were visible in the ultrasound examination. Contrast-enhanced computed tomography showed thrombosis of the portal and hepatic veins and suspected carcinoma in S3/4 of the liver (size of lesion: 14×13 mm). The patient underwent transjugular intrahepatic portosystemic shunt (TIPS) and transhepatic arterial chemotherapy and embolization (TACE). One month later, the patient’s abdominal distension had resolved and there was no sign of ascites. Laboratory test results showed a significant increase in serum albumin, and there was no remission of proteinuria (serum albumin, 34.4 g/L; proteinuria, 5.3 g/24 hours; uRBC, 175/µL; protein-to-creatinine ratio, 4,176 mg/g). The timeline of the clinical course is shown in Figure 1.
Discussion and conclusions
IgAN is the most common glomerulonephritis in the world, especially in the Asian population (8). In this case, we believe that IgAN was secondary to liver cirrhosis and HCV infection rather than a primary disease for three reasons: first, partial remission was achieved after eradication of HCV; second, IgAN constitutes 36% to 60% of renal pathology in liver cirrhosis (4,9), and a membranoproliferative pattern of injury was reported in patients with chronic liver disease (10); third, relapse occurred when liver cirrhosis and portal hypertension progressed. Recently, immunostaining of galactose-deficient IgA1 (Gd-IgA1) with KM55 antibody in kidney histology has been proposed to be a novel method to differentiate primary IgAN (11). Some studies showed that Gd-IgA1 staining was less intensive in other glomerulonephritis with IgA deposits-lupus nephritis. However, Gd-IgA1 staining with current methods is not specific enough to be a diagnostic tool in clinical routine (12). More study is needed for IgAN biomarkers.
HCV-associated glomerulonephritis is an important extrahepatic manifestation of HCV infection which often occurs with mixed cryoglobulinemia (13). MPGN with IgG, IgM, and C3 deposits is the most common pathological type (2), while IgAN has also been reported in a few cases (14). In this case, to some extent, we considered the IgAN to be secondary to the HCV infection because partial remission occurred after eradication of the virus. The absence of HCV antigen in kidney specimens may be due to the low sensitivity of immunohistochemistry examination, as previously reported (15). The most probable explanation of NS relapse despite an SVR was the progression of liver cirrhosis and portal hypertension which was supported by the presence of new-onset ascites, esophageal and gastric varices aggravation, portal and hepatic veins thrombosis, and hepatic carcinoma. We believe that diabetes mellitus did not contribute to the NS in this case because no feature of diabetic nephropathy was noted in kidney histology, no diabetic retinopathy was found in fundus photography, and the patient had a good glycemic control.
The main treatment for HCV in the context of liver cirrhosis and CKD is DAA drugs. Sofosbuvir plus ledipasvir for 12 weeks is recommended by the Kidney Disease: Improving Global Outcomes (KDIGO) and American Association for the Study of Liver Diseases (AASLD) guidelines for genotype 1b HCV infection when eGFR is more than 30 mL/minute (16,17). We did not initially prescribe DAA drugs concurrent with immunosuppressive agents because they were not available in China in February 2015. However, the delay in DAA treatment in this case provided an insight about its role in managing IgAN secondary to HCV liver cirrhosis.
In severe renal impairment, the sofosbuvir-based regimen was once contraindicated because its metabolite is eliminated through the kidney. However, recent research has demonstrated the safety of sofosbuvir (18), and it has been approved by the US Food and Drug Administration (FDA) to be used in late-stage CKD patients. In our case, the patient developed AKI after initial management. We believe that the AKI did not result from progression of IgAN. Instead, it could be caused by drugs such as irbesartan and aggressive diuretics or by the serious fungal infection that was induced by immunosuppressive treatment. We prescribed sofosbuvir 400 mg and ledipasvir 90 mg once daily for the patient when she had a low eGFR and was on dialysis, because new DAA drugs (i.e., glecaprevir and grazoprevir) were not available worldwide. Furthermore, the patient’s kidney function was recovering. After the 12-week treatment, no sofosbuvir-associated adverse events, including a decrease in eGFR, were noted.
Research on the treatment of biopsy-proven IgAN secondary to HCV liver cirrhosis is rare because of the higher complication risk associated with the biopsy procedure and the relatively benign renal manifestations including asymptomatic proteinuria and microscopic hematuria. However, we reported the case of a patient with IgAN with a membranoproliferative pattern of injury secondary to decompensated HCV cirrhosis with 5-year follow-up. Partial remission was achieved after treatment using DAA drugs. The main limitation of our report is the lack of kidney pathology after the NS relapse and a lack of data on immunofluorescence and HCV antigen because they were not routinely recorded.
In conclusion, our case suggests that DAA drugs may be used to treat patients with IgAN with a membranoproliferative pattern of injury in decompensated HCV cirrhosis, although relapse could occur with the progression of liver cirrhosis despite an HCV SVR.
Acknowledgments
The authors would like to acknowledge the patient and her family for providing their information and giving consent to its publication. The authors would also like to thank Zixin Sybil Sha for her help in polishing this paper.
Funding: This work was supported by “Guangdong Basic and Applied Basic Research Foundation” (No. 2020A151501292).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-21-5289/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-21-5289/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Park H, Chen C, Wang W, et al. Chronic hepatitis C virus (HCV) increases the risk of chronic kidney disease (CKD) while effective HCV treatment decreases the incidence of CKD. Hepatology 2018;67:492-504. [Crossref] [PubMed]
- El-Serag HB, Hampel H, Yeh C, et al. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology 2002;36:1439-45. [Crossref] [PubMed]
- Dey AK, Bhattacharya A, Majumdar A. Hepatitis C as a potential cause of IgA nephropathy. Indian J Nephrol 2013;23:143-5. [Crossref] [PubMed]
- Hemminger J, Arole V, Ayoub I, et al. Acute glomerulonephritis with large confluent IgA-dominant deposits associated with liver cirrhosis. PLoS One 2018;13:e0193274. [Crossref] [PubMed]
- van Marck EA, Deelder AM, Gigase PL. Effect of partial portal vein ligation on immune glomerular deposits in Schistosoma mansoni-infected mice. Br J Exp Pathol 1977;58:412-7. [PubMed]
- Ghabra N, Piraino B, Greenberg A, et al. Resolution of cirrhotic glomerulonephritis following successful liver transplantation. Clin Nephrol 1991;35:6-9. [PubMed]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Health Med 2013;2:38-43. [Crossref] [PubMed]
- McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant 2011;26:414-30. [Crossref] [PubMed]
- Nakamoto Y, Iida H, Kobayashi K, et al. Hepatic glomerulonephritis. Characteristics of hepatic IgA glomerulonephritis as the major part. Virchows Arch A Pathol Anat Histol 1981;392:45-54. [Crossref] [PubMed]
- Andeen NK, Jefferson JA, Akilesh S, et al. IgA-dominant glomerulonephritis with a membranoproliferative pattern of injury. Hum Pathol 2018;81:272-80. [Crossref] [PubMed]
- Yasutake J, Suzuki Y, Suzuki H, et al. Novel lectin-independent approach to detect galactose-deficient IgA1 in IgA nephropathy. Nephrol Dial Transplant 2015;30:1315-21. [Crossref] [PubMed]
- Zhao L, Peng L, Yang D, et al. Immunostaining of galactose-deficient IgA1 by KM55 is not specific for immunoglobulin A nephropathy. Clin Immunol 2020;217:108483. [Crossref] [PubMed]
- Ferri C, Greco F, Longombardo G, et al. Antibodies to hepatitis C virus in patients with mixed cryoglobulinemia. Arthritis Rheum 1991;34:1606-10. [Crossref] [PubMed]
- Adebajo CO, Sathick IJ, Garovic VD. 63-year-old man with chronic hepatitis C virus infection and proteinuria. Mayo Clin Proc 2013;88:e93-e7. [Crossref] [PubMed]
- Cao Y, Zhang Y, Wang S, et al. Detection of the hepatitis C virus antigen in kidney tissue from infected patients with various glomerulonephritis. Nephrol Dial Transplant 2009;24:2745-51. [Crossref] [PubMed]
- Ghany MG, Morgan TR. AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C guidance 2019 update: American association for the study of liver diseases-infectious diseases society of america recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology 2020;71:686-721. [Crossref] [PubMed]
- Kidney disease: improving global outcomes (KDIGO) hepatitis C work group. KDIGO 2018 clinical practice guideline for the prevention, diagnosis, evaluation, and treatment of hepatitis c in chronic kidney disease. Kidney Int Suppl (2011) 2018;8:91-165. [Crossref]
- Borgia SM, Dearden J, Yoshida EM, et al. Sofosbuvir/velpatasvir for 12 weeks in hepatitis C virus-infected patients with end-stage renal disease undergoing dialysis. J Hepatol 2019;71:660-5. [Crossref] [PubMed]


