Precision medicine in spinocerebellar ataxias: treatment based on common mechanisms of disease
Introduction
Spinocerebellar ataxias (SCAs) are a heterogeneous group of dominantly inherited neurological disorders associated with degeneration of neurons in the cerebellum and its associated pathways. Although SCAs are generally characterized by symptoms of cerebellar dysfunction, such as unsteady gait, uncoordinated limb movements, and slurred speech, individual SCAs have variable involvement of extra-cerebellar areas of the nervous system. There is also great variability in the age of onset and rate of disease progression in the individual SCAs. Additionally, symptoms vary even in different individuals harboring the same disease-causing SCA mutation. This phenomenon can be most clearly seen in the polyglutamine ataxias (SCAs 1, 2, 3, 6, 7, and 17), diseases caused by an expanded CAG repeat sequence, which encodes glutamine, in the disease-causing gene (1). In these cases, individuals with similar repeat sizes can have significantly different ages of disease onset, rate of disease progression, and involvement of the cerebellum and other areas of the nervous system (2). This suggests that modifiers of disease exist and play a substantial role in disease onset and progression.
One obstacle to developing precise treatments for SCAs is the diversity of causes of the condition. Mutations in more than 30 genes can result in SCA and, while the affected genes are known for many etiologies, the overlap in clinical symptoms makes accurate early diagnosis difficult (1). When broadened to include all forms of cerebellar ataxia, there are likely hundreds of disease-causing mutations. Despite the numerous causes for cerebellar ataxia, no effective symptomatic or disease-modifying therapies currently exist. While improvements in diagnostic strategies including whole exome sequencing (3,4) will increase the likelihood of identifying specific disease-causing mutations, the development of precise therapies for SCAs is challenging given the diverse genetic causes of SCA and variability in clinical features even in a defined genetic cause. Two approaches are possible for the development of these treatments: (I) the use of gene-targeting strategies for specific disease-causing mutations; and (II) the identification of convergent mechanisms of dysfunction as the basis for treatment. This review will highlight current progress on these two therapeutic approaches. The identification and targeting of common mechanisms of neuronal dysfunction is likely to be a more effective and realistic therapeutic strategy in the short term. We identify mechanisms of dysfunction including aberrant protein homeostatic pathways, RNA toxicity, altered Purkinje neuron membrane physiology, and intracellular calcium handling as potential pathogenic mechanisms that are shared across the different SCAs, and how precisely targeting these pathways may treat different etiologies of SCA.
Approach I: treating SCA through gene suppression strategies
Many SCAs, including the polyglutamine ataxias, are caused by autosomal-dominant gain-of-function mutations. Gene suppression strategies, such as RNA interference and antisense oligonucleotides, are an attractive option for the treatment of gain-of-function mutations (5-7).
Strategies which target disease-causing alleles have been effective in multiple SCA models. Adeno-associated viral delivery of short hairpin RNAs (shRNAs) against the mutant human ataxin-1 gene improved Purkinje neuron morphology and motor function in a mouse model of SCA1 (8), while both AAV-mediated overexpression of wild-type ataxin-1 and miRNA against ataxin-1 have recently been shown to be effective (9). Furthermore, shRNA against mutant ataxin-1 has been effectively delivered to deep cerebellar nuclei in order to transduce Purkinje neurons, resulting in improved cerebellar Purkinje neuron morphology and motor function (10). Recently, small interfering RNA (siRNA) designed to non-specifically silence both mutant and wild-type ataxin-7 was delivered in AAV2/1 shuttle vectors in a mouse model of SCA7. This strategy reduced nuclear inclusions and improved Purkinje neuron molecular layer thickness, demonstrating the potential efficacy of knocking down overall levels of ataxin-7 at improving the disease phenotype (11). Allele-specific silencing of mutant ataxin-7 was previously shown in patient derived fibroblasts (12). However, a similar strategy of injecting an AAV which encodes a microRNA-like molecule directed against mutant ATXN3 did not improve motor function in SCA3 mice, although the treatment was effective at reducing mutant ataxin-3 protein levels (13). Interestingly, antisense oligonucleotides which are designed to promote skipping of exon 9 (the polyglutamine-containing exon) of the ATXN3 gene can effectively limit the presence of this exon in control mice (14). Taken together, these studies indicate that both RNA interference-based and antisense oligonucleotide-based strategies to silence specific alleles may have relevance for the treatment of polyglutamine SCAs.
It may be possible to use RNA interference strategies to target multiple polyglutamine expansion disorders through a single construct. Antisense oligonucleotides which have been modified to specifically target expanded polyglutamine sequences can effectively reduce mRNA and protein expression of mutant huntingtin, mutant ataxin-1, and mutant ataxin-3 in patient-derived fibroblasts (15). This study corroborates prior work indicating that antisense oligonucleotides designed to target expanded CAG repeats are effective in specifically silencing mutant ataxin-3 and mutant huntingtin alleles in cultured cells (16). These studies indicate that expanded CAG repeat regions are potential targets for gene-suppression strategies in polyglutamine ataxias.
Although gene-targeting strategies are attractive for the precise treatment of SCAs which result from gain-of-function mutations, the development of targeted treatments for each and every etiology of SCA is not currently feasible. Over 30 known mutations result in SCA (1), and many other disease-causing mutations likely remain undiscovered. Gene-targeting strategies and, particularly, delivery options are likely not economically viable in the short term. Identifying common, treatable mechanisms of neuronal dysfunction which contribute to disease are likely to be more effective for the treatment of diverse causes of SCA.
Approach II: search for convergent mechanisms of disease for the treatment of SCAs
Altered protein homeostasis
In several of the SCAs, aberrant protein folding likely plays a significant role in disease pathogenesis. This phenomenon of misfolding is particularly relevant for the polyglutamine ataxias, since expanded glutamine repeat sequences are particularly prone to misfolding events and seem to have toxic effects in certain neuronal populations (17,18). Proteins harboring expanded glutamine repeat sequences form large neuronal inclusions and smaller oligomeric protein products found throughout the cell. Evidence suggests that large inclusions, once thought to be central to neuronal toxicity in polyglutamine diseases, may in fact be a normal byproduct of protein quality control mechanisms and are possibly representative of a protective cellular response (19-21). Alternatively, small polyglutamine protein oligomers, and the cellular processes which are altered upon their formation, may be a larger cause of neuronal toxicity in polyglutamine ataxia (17,22,23). These features of disease are outlined in Figure 1.
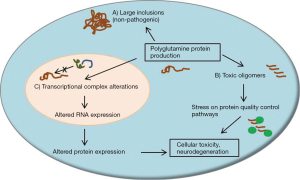
Molecular chaperone pathways play a large role in protein homeostasis and are altered in several neurodegenerative protein misfolding diseases. These pathways include the heat shock proteins (Hsp), which are likely involved in the pathogenesis of neurodegenerative disorders (17,24). Molecular chaperone pathways seem to have relevance for the pathogenesis of multiple etiologies of SCA. SCA7 patient-derived lymphoblastoid cells show reduced expression of Hsp70 and Hsp27 with no change in Hsp90 levels (25), and SCA3 patient-derived fibroblasts display a reduction in Hsp40 levels which correlates with age-of-onset (26). In cell-based assays, expression of mutant PKC gamma, which results in SCA14, is associated with an increase in Hsp70, which may be a protective response to reduce elevated cell stress due to the presence of mutant protein oligomers. Knockdown of Hsp70 increased protein aggregation and decreased survival in this assay, suggesting that the Hsp70 pathway may be important for protein quality control in SCAs (27).
Since neurodegeneration improves upon Hsp70 activation or overexpression in some models of protein folding disease, Hsp70 activators may be therapeutic options for the treatment of human SCAs (28,29). Recently, Hsp70 levels have been shown to influence neuronal survival by facilitating transcription of mesencephalic astrocyte-derived neurotrophic factor (MANF) in a knock-in mouse model of SCA17 (30). Hsp70 expression levels are progressively decreased in this model and correlate with the onset and severity of motor impairment and neurodegeneration. In cell-based assays, this was shown to be due in part to a decreased interaction of TATA-box-binding protein and XBP1s, a transcription factor, resulting in decreased MANF expression. Increasing Hsp70 levels improved the interaction between TATA-box-binding protein and XBP1s, which improved MANF transcription, and independently increasing MANF expression in vivo decreased neurodegeneration (30). Levels of several other chaperones, including Hsp27 and the co-chaperone sacsin, are reduced in other SCA animal models, further indicating that molecular chaperones are targets for pharmacologic intervention in SCA (31-33).
Overall, restoring balance to protein homeostatic pathways may have great benefits for the protein folding disorders such as the polyglutamine ataxias. One proposed treatment strategy for polyglutamine-induced neurodegeneration is the use of Hsp90 inhibitors to force a greater proportion of misfolded proteins to the proteasome for substrate degradation (34,35). Hsp70 activators may promote clearance and degradation of polyglutamine proteins (35). Additionally, Hsp27 induction was associated with gene transcription in cells transfected with mutant TATA-box-binding protein, the mutation which causes SCA17 (36). Hsp27 promoted transcription of the tyrosine kinase receptor TrkA, which is important for nerve growth factor (NGF) signaling and promotes neuronal survival. Therefore, Hsp27 activation may also have neuroprotective benefits in SCAs (36). Modulators of Hsp function, and other strategies which limit the stress placed on protein homeostatic pathways by polyglutamine expanded proteins, may have great benefits for the treatment polyglutamine ataxias and, potentially, other SCA etiologies which involve mutations which result in the accumulation of misfolded proteins.
Transcriptional dysregulation
Several SCAs are associated with altered gene transcription through the disruption of transcriptional complexes (Figure 1). For example, SCA17 is a result of a polyglutamine expansion in TATA-box-binding protein (TBP) (37,38). Disruption of normal TBP function reduces the interaction with the transcription factor TFIIB, thereby reducing the expression of genes under normal TBP control (39,40). One such gene is TrkA, a tyrosine kinase receptor for NGF and undergoes reduced expression in the presence of mutant TBP (41). Similarly, SCA7 results from a polyglutamine expansion of the ATXN7 gene, which encodes the ataxin-7 protein, a member of the STAGA complex which is a transcriptional regulator possessing both acetyltransferase and deubiquitinase activity. Although it is still unclear what the exact role polyglutamine expansion has on STAGA function, the complex may disassociate, become rendered inactive, or experience increased deubiquitinase or acetyltransferase activity (42). At least one mouse model has illustrated that polyglutamine expansion in the ataxin-7 protein has profound effects on gene transcription (43). Finally, evidence also suggests that SCA1 disease pathogenesis is at least partially due to altered protein complex formation, as ataxin-1 plays an important role in both an RNA binding complexes containing RBM17 or U2AF65 (44,45) and a transcriptional repression complex containing capicua (46,47).
Direct alterations in transcriptional complex formation can influence the transcription of downstream gene targets. There is evidence that altered histone deacetylase (HDAC) activity accompanies pathology in several polyglutamine SCAs. In a mouse model of SCA3, histones H3 and H4 were found to be hypo-acetylated, suggesting that HDAC overactivity may be present. Indeed, the HDAC inhibitor sodium butyrate improved histone acetylation and increased the expression of genes suppressed in SCA3 mouse cerebellum. Additionally, sodium butyrate improved motor function and survival in SCA3 mice (48). Ataxin-7 has been shown to interact directly with HDAC3 in transfected HEK293T cells, possibly promoting toxicity through altered deacetylation in a polyglutamine-dependent manner. Indeed, HDAC3 protein levels were increased in the cerebellum of SCA7 mice, suggesting that HDAC3 inhibition may be a therapeutic target (49). However, one recent study suggests that a cautious approach should be taken to HDAC inhibition as a therapy for SCAs. SCA1 mice were crossed with Purkinje-neuron specific HDAC3-null mice in order to determine whether HDAC inhibition may improve motor impairment. Surprisingly, while haploinsufficiency of HDAC3 did not improve disease progression, full knockout of HDAC3 worsened motor impairment and neurodegeneration in SCA1 mice (50). This suggests that HDAC3 function may be essential for normal Purkinje neuron function and therefore be a poor choice for therapeutic targeting.
Taken together, even though HDAC inhibitors may have therapeutic promise for the treatment of some forms of SCA, particularly those which display repressed gene transcription, further studies are needed to determine the specific classes of HDAC which contribute most significantly in ataxia and whether their inhibition will be a safe therapeutic option.
RNA toxicity
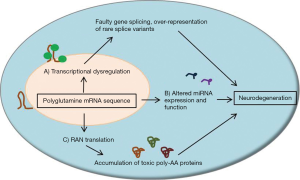
While polyglutamine protein toxicity and disrupted protein homeostasis is thought to play a major causative role in the progression of SCAs, transcribed RNA products of expanded CAG repeat sequences may play an independent role in cellular toxicity. Structurally, repeat sequences within transcribed RNA regions, including CAG repeat sequences, tend to form hairpins of varying size and stability (51). These hairpin structures likely play specific roles in the cellular processing of RNA strands, such as recruiting certain RNA-binding proteins to influence translation or degradation, but the roles of polyglutamine repeat regions within RNA transcripts are not fully known. However, it is plausible that expanded polyglutamine repeat sequences can alter mRNA homeostasis in a manner similar to the disruption of protein homeostasis discussed previously. It has been proposed that expanded CAG repeat sequences can recruit high levels of RNA splicing factors and transcription factors, leading to an abnormal expression of rare splice variants of other mRNAs (52). The resultant altered gene expression may lead to neuronal dysfunction and influence neurodegeneration.
Disease models and human mutations support a toxic RNA hypothesis of neurodegeneration in SCAs. In a model of SCA3, transcribed polyglutamine RNA sequences were sufficient to induce neurodegeneration even in the absence of polyglutamine protein (53). In humans, SCA10 results from a large pentanucleotide sequence of AAUCU, localized to intron 9 of the ATXN10 gene. Even though this expanded pentanucleotide repeat sequence is non-coding, RNA aggregates were seen in affected cells and were associated with toxicity (54). Large microsatellite repeat regions localized to introns of the TK2 gene, which encodes thymidine kinase 2, or BEAN (brain expressed, associated with Nedd4) cause SCA31. Expanded pentanucleotide repeats of TGGAA are associated with SCA31 and, since they are localized to non-coding regions of DNA, may also alter splicing or result in toxic mRNA production by forming RNA aggregates in nuclei (55). This suggests that RNA toxicity alone, even in the absence of translated protein, can result in neurodegeneration in SCAs.
A novel repeat-associated non-AUG (RAN) translation has recently been described for repeat containing RNA. In RAN translation, repeat-containing RNA strands are translated in multiple reading frames, thereby producing up to three different protein products when occurring within a polyglutamine repeat sequence (56,57). In SCA8, it has been reported that transcriptional initiation of the ATXN8/ATXN8os gene can occur in all three reading frames, resulting in not only poly-glutamine proteins but also poly-alanine and poly-serine proteins (56,57). These protein products occur even though the expanded polyglutamine sequence is located in a non-coding region of the ATXN8/ATXN8os gene (58), suggesting a toxic RNA gain-of-function mutation (59,60). Interestingly, all three of these transcribed protein products were shown to be present in the same cell, suggesting that these processes can be active simultaneously, and that these protein products are present in cerebella from both mouse models of SCA8 and human SCA8 patients (57). RAN products may play unknown roles in other SCAs, so they should be considered potential therapeutic targets.
Recently, altered microRNA (miRNA) function has been noted in multiple models of SCA. Aberrant miRNA expression has been shown to influence disease throughout the body, including the brain (61). Knockout of Dicer1, a gene which is necessary for the normal production and expression of miRNAs, causes neurodegeneration in mouse cerebellum (62,63). Several miRNA variants are likely important in neurological disease. miR-19, miR-101, and miR-130 have been shown to regulate ATXN1 levels and likely influence disease in a mouse model of SCA1 (64). A recent study found that aberrant cross-regulation between miR-124 and long non-coding RNA associated with the ATXN7 gene may directly impact gene transcription and neurodegeneration in both cerebellum and retina of SCA7 mice (65). Additionally, the ataxin-2 protein is involved in miRNA pathways in a Drosophila model (66). Future studies will need to determine the prevalence of miRNA dysfunction in other SCA etiologies in order to consider their potential as therapeutic targets.
Overall, RNA toxicity likely plays a role in the disease progression of SCAs and may directly influence neuronal dysfunction and neurodegeneration (Figure 2). Therapies which limit splicing factor and transcription factor recruitment to expanded CAG repeat sequences, along with agents which limit RAN translation or aberrant miRNA expression, may have a role in the treatment of SCAs.
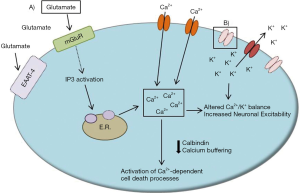
Aberrant intracellular calcium handling
Calcium homeostasis is central to proper neuronal function. As a mediator of many cellular processes, cytosolic calcium levels are tightly regulated in neurons by a number of calcium buffers and transporters. For example, the calcium buffers parvalbumin and calbindin are particularly enriched in Purkinje neurons, perhaps to account for the large levels of calcium influx associated with spontaneous, repetitive action potential firing and increased metabolic load in these neurons. Neuronal calcium transients can come from a variety of sources, including intracellular calcium release stores upon synaptic metabotropic glutamate receptor (mGluR) activation and through voltage-gated calcium channels throughout the Purkinje neuron membrane during cycles of depolarization.
Alterations in calcium entry and intracellular calcium regulation are known causes of human ataxia. ITPR1 mutations cause SCA15 and likely contribute to imprecise synaptic signaling (67,68). The mutation in ITPR1, the inositol 1,4,5-trisphosphate receptor, may result in neurodegeneration by preventing the normal intracellular calcium release from occurring upon excitatory synaptic input (67). Additionally, SCA14 results from a mutation in protein kinase C gamma, which has been proposed to lead to alterations in intracellular calcium homeostasis and impaired Purkinje neuron responses to synaptic input (69,70). Finally, CACNA1A mutations result in both SCA6 and episodic ataxia type 2, suggesting that improper calcium entry through voltage-gated calcium channels can influence Purkinje neuron function and induce neurodegeneration (71,72).
Altered Purkinje neuron calcium handling is a prominent feature in animal models of SCA (73). In a mouse model of SCA1, the intracellular calcium-release channels ITPR1 and SERCA3 are expressed at lower levels in the cerebellum at the onset of motor impairment, a feature which was also noted in human patient tissue samples (74). The down-regulation of these channels may be in response to an impaired ability of the Purkinje neuron to control intracellular calcium levels, since calbindin D-28K is also reduced in SCA1 mouse cerebellum (75). More evidence for this hypothesis comes from a recent study which showed that suppression of calbindin D-28K worsens motor performance in SCA1 mice, indicating that impaired calcium buffering capacity may be an important mechanism of neuronal dysfunction in Purkinje neurons (76). Additionally, knockout of the acid-sensing ion channel isoform 1a (ASIC1a), a channel which passes inward calcium current upon activation, also improves motor performance in SCA1 mice and improves dendritic morphology, calbindin staining intensity, and parvalbumin staining intensity (77). Together, these studies indicate that SCA1 Purkinje neurons are particularly sensitive to perturbations in intracellular calcium levels, and that intracellular calcium handling capacity is likely impaired due to altered expression of calbindin D-28K protein. Reduced calbindin levels have also been noted in mouse models of SCA2 and SCA7, suggesting that this may be a common feature of SCAs (33,78).
In a series of studies, polyglutamine-expanded ataxin-2 and polyglutamine-expanded ataxin-3 proteins have been shown to interact directly with the inositol 1,4,5-trisphosphate receptor, thereby increasing sensitivity to inositol 1,4,5-trisphosphate and facilitating intracellular calcium release (79,80). In SCA2 mice, suppression of intracellular calcium release was achieved by expression of inositol 1,4,5-phosphatase, an enzyme which reduces the levels of intracellular inositol 1,4,5-trisphosphate. This strategy improved motor performance and, surprisingly, improved Purkinje neuron firing properties (81).
Taken together, it is likely that aberrant intracellular calcium handling is present in multiple SCAs (Figure 3). Treatments which increase expression of calcium binding proteins, such as calbindin, or inhibit intracellular calcium release stores may be one strategy to improve neuronal health and limit calcium-mediated neuronal toxicity in SCAs.
Synaptic alterations
Purkinje neurons undergo plasticity at the parallel fiber synapse in response to excitatory synaptic input. Classically, long-term depression (LTD) at the parallel fiber synapse has been associated with cerebellar learning. In particular, calcium entry upon mGluR activation and TRPC3 channel opening, along with subsequent activation of inositol 1,4,5-trisphosphate, allows LTD to occur at the parallel fiber synapse (82), while glutamate re-uptake at the Purkinje neuron presynaptic cleft is extremely important for precise signaling and is controlled by the glutamate transporters EAAT-4, GLT-1, and GLAST (83). Altered glutamatergic input strength and precision could have profound effects on Purkinje neuron physiology and contribute to the generation of ataxia. For example, SCA5 is caused by a mutation in the SPTBN2 gene, which encodes the beta-III spectrin protein (84). Beta-III spectrin stabilizes EAAT-4 at the Purkinje neuron membrane, indicating that loss-of-function mutations in SCA5 likely contribute to excess synaptic glutamate load and subsequent postsynaptic glutamate toxicity.
In mouse models of SCA1, several synaptic proteins show reduced expression by mid-stage disease and likely play a role in altered synaptic function. Through comparative microarrays, one study identified transcriptional changes in the presence of mutant ataxin-1 protein. Results from this analysis point to synaptic dysregulation as a likely source of altered Purkinje neuron physiology (75). In addition to reduced expression of the calcium-binding proteins mentioned previously, EAAT-4 reduction is seen in SCA1 mouse cerebellum early in disease (74,75). While synaptic calcium transients were shown to be unaffected in SCA1 mice upon glutamate stimulation, secondary intracellular calcium release was prolonged (85). This suggests that an interaction between synaptic calcium entry and altered intracellular calcium handling may be present and important for cellular excitability in SCA1 mice.
Glutamate toxicity has been proposed to play a role in several other mouse models of SCA. Non-cell autonomous Purkinje neuron degeneration has been demonstrated in multiple models of SCA7 (86,87). Impaired Bergmann glial glutamate transport appears to play a role in disease progression in at least one of these models, suggesting that synaptic dysregulation contributes to Purkinje neuron dysfunction in SCA7 (86). Additionally, application of the mGluR1/5 agonist DHPG induces larger intracellular calcium transients in both cultured rat medium spiny neurons transfected with polyglutamine ataxin 3 and cultured Purkinje neurons from SCA2 mice (79,80). Direct glutamate application was also shown to cause increased neuronal death in cultured SCA2 Purkinje neurons, suggesting that synaptic glutamate toxicity may be a source of calcium-induced neuronal death in SCAs (79). Furthermore, inhibiting downstream calcium release with the IP3 receptor inhibitor dantrolene improved motor performance in both SCA2 and SCA3 mice, and molecular layer thickness in SCA2 mice (79,80). This suggests that synaptic dysregulation and intracellular calcium levels are likely closely tied in SCAs.
Overall, excess synaptic glutamate load, combined with a reduced ability of the Purkinje neuron to buffer intracellular calcium, increases neuronal excitability and likely influences excitotoxic cell death in multiple SCAs (Figure 3). Drugs which improve glutamate uptake or reduce intracellular calcium release will likely improve synaptic physiology in these SCAs. Dantrolene and glutamate transport activators may have therapeutic potential in this capacity.
Altered Purkinje neuron membrane excitability
Altered neuronal physiology within the olivo-cerebellar circuit can result in ataxia (88). In humans, mutations in the KCNC3 gene lead to Purkinje neuron degeneration in SCA13 through a loss in voltage-gated potassium channel function and, presumably, neuronal hyperexcitability (89,90). SCA19/22 is a result of a mutation in the KCND3 gene, which encodes for the voltage-gated potassium channel Kv4.3 (91,92). SCA6 and episodic ataxia type 2 both result from mutations in CACNA1A, which likely influences both neuronal calcium entry and firing properties (71,72).
Mouse models have outlined how ion channel dysfunction, either through mutations or altered expression levels, are sufficient to induce ataxia through disrupted Purkinje neuron firing. In mouse models of episodic ataxia type 2, irregular Purkinje neuron pacemaking results from mutations in the CACNA1A gene, which encodes a P/Q-type voltage-gated calcium channel, and results in motor impairment (93-96). Activators of small-conductance calcium-activated potassium (SK) channels, 1-EBIO and chlorzoxazone, both improve the regularity of Purkinje neuron firing and improve motor impairment in these mice (93,97). These studies highlight the importance of regular Purkinje neuron pacemaking for motor function, and that correcting Purkinje neuron electrophysiologic dysfunction can have beneficial effects on motor performance.
In several mouse models of SCA, Purkinje neuron electrophysiologic dysfunction correlates with the onset of motor impairment and early stages of neurodegeneration. The findings from these studies suggest that electrophysiologic dysfunction may contribute to pathogenesis early in disease, and that correcting this dysfunction may be beneficial for prolonging normal motor function and slowing neurodegeneration. In a mouse model of SCA1, progressively reduced Purkinje neuron firing frequency was noted alongside an increased density of surface Kv4.3 channel expression. Use of 4-aminopyridine (4-AP), a compound which blocks voltage-gated potassium channels, increased simple spike firing frequency and improved motor performance, suggesting that the SCA1 phenotype may be partially explained by a reduced Purkinje neuron firing rate (98). This result agrees with a previous study, which illustrates that Purkinje neurons from SCA1 mice have a delayed onset to the initial spike in a depolarizing current injection protocol, and this effect is suppressed by either 4-AP administration or a hyperpolarizing prepulse (85).
Recently, in the same mouse model of SCA1, altered Purkinje neuron membrane properties were shown to result from a reduction in expression and function of two potassium channels, the large-conductance calcium-activated potassium (BK) and G-protein coupled inwardly-rectifying potassium type 1 (GIRK1) channels, which resulted in membrane depolarization. BK and GIRK1 channels showed a progressive reduction over the course of disease, and increasing BK channel expression through an adeno-associated virus improved motor performance and reduced dendritic degeneration (99). These findings suggest that altered Purkinje neuron excitability in SCA1 mice can be attributed to an imbalance of depolarizing and hyperpolarizing currents due to altered potassium channel function.
Altered membrane physiology has been noted in several other models of SCA. In SCA6 mice, polyglutamine expansion of the CACNA1A gene results in decreased current density of P/Q-type calcium channels in dissociated Purkinje neurons (100). Additionally, in mice lacking beta-III spectrin, the gene which results in SCA5 in humans, Purkinje neurons have a reduced firing rate which may be due in part to a reduction in voltage-gated sodium channel current (101). These findings indicate that a subset of SCAs may contain mutations which decrease Purkinje neuron excitability. It will be important to determine how neuronal excitability is altered in different etiologies of SCA, in order to develop pharmacologic strategies which target altered neuronal firing.
SK channel activators are particularly intriguing candidate compounds, as activating SK channels has been shown to restore membrane physiology and reduce neurodegeneration in several animal models of SCA. In a model of SCA2, a progressive reduction in firing frequency was noted in parallel with increased severity of neurodegeneration and increasing levels of motor impairment (78). No alterations in ion channel expression were found to accompany this change in physiology, but an exhaustive analysis of ion channels was not performed. In another SCA2 mouse model, treatment with the SK channel activators CyPPA, NS309, and NS13001 improved Purkinje neuron firing regularity and improved motor performance (102). SK channel activating compounds were also shown to be effective in a mouse model of SCA3, where alterations in voltage-gated potassium channel activation and deactivation contribute to altered Purkinje neuron firing patterns and increased neuronal excitability (103). Finally, SK channel activators also improve pacemaking regularity and normalize neuronal excitability in mouse models of ataxia due to CACNA1A mutations, suggesting that compounds which activate SK channels may have profound effects on improving Purkinje neuron function in SCA etiologies which involve altered Purkinje neuron pacemaking (97,104).
Among the compounds likely to effectively treat multiple etiologies of SCA, SK channel activators seem to have particular promise. Recently, a clinical trial for the drug riluzole was shown to be effective for the symptomatic treatment of several etiologies of autosomal dominant SCA and Friedrich’s ataxia (105,106). The trial suggests that common mechanisms of dysfunction may exist across different etiologies of human SCA. Although the mechanism of action of riluzole in this study is unclear, among its other actions, riluzole is an SK channel activator (107). Agents which have greater specificity for SK channels than riluzole have been developed and may be more effective for the treatment of SCAs (108).
Together, these studies suggest that altered Purkinje neuron excitability, possibly due to altered potassium and calcium channel function, may be a common mechanism of neuronal dysfunction in the SCAs (Figure 3). Potassium channel modulators, and particularly SK channel activators, appear to be good candidates for treating neuronal dysfunction across different causes of SCA.
Strategy for intervention
SCAs are relentlessly progressive, debilitating diseases. In order to design treatment strategies which are optimally effective for SCAs, it will be necessary to improve diagnosis during presymptomatic stages. Presymptomatic diagnosis will maximize the benefit of treatments by allowing early treatments to slow or halt disease progression before extensive neurodegeneration has occurred. This will improve the possibility that connectivity between neuronal populations in the olivo-cerebellar circuit can be preserved, thereby helping SCA patients maintain motor function. Trials like RISCA, which is focused on the identification and proper diagnosis of cerebellar ataxia in presymptomatic cerebellar ataxia patients, are essential for the treatment of these diseases (109). Additionally, presymptomatic diagnoses will improve the ability of clinical trials to determine the efficacy of therapeutics by offering the earliest possible stage of intervention.
Conclusions
While many ataxia-causing mutations are currently known, there are many others which remain unidentified. Targeting convergent mechanisms of neuronal dysfunction in ataxia appears to be the most effective therapeutic intervention in the near future. Toxic protein oligomers can potentially be reduced through the activation of Hsp family members, while reducing RNA aggregation and transcriptional dysregulation may also have beneficial effects on disease progression. However, compounds which effectively target these pathways are not well characterized or remain unknown. Alternatively, compounds which limit Purkinje neuron excitability and reduce intracellular calcium release have proven beneficial in multiple models of SCA. These compounds, such as SK channel activating compounds and dantrolene, could improve Purkinje neuron function by normalizing pacemaker firing and could reduce the activation of calcium-dependent cell death processes leading to neurodegeneration. The success of riluzole in clinical trials indicates that SK channel activation may have particular benefits for the treatment of multiple etiologies of SCA.
In the long term, gene targeting strategies may be best for the specific targeting of dominant mutations which cause cerebellar ataxias. However, gene delivery options are currently cumbersome and are associated with significant risk. With the wide range of disease-causing mutations in SCAs, common mechanisms of Purkinje neuron dysfunction are the most attractive target for widespread treatment of SCAs. More studies are necessary to determine whether shared mechanisms of disease truly exist across all ataxias, and whether the selective vulnerability of cerebellar neurons may be due to their unique metabolic and electrophysiologic properties. Targeting aberrant neuronal physiology, faulty protein and mRNA homeostasis, and calcium dysregulation is the most accessible form of treatment available for cerebellar ataxia patients in the near future, and efforts should be taken to design and test widely applicable therapies for SCA which are designed to target these features of disease.
Acknowledgements
Funding was provided through the NIH, K08NS072158 (VG Shakkottai), R01NS085054 (VG Shakkottai), DoD-Army PR1215668 (GG Murphy), and R01AG028448 (GG Murphy).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol 2010;9:885-94. [PubMed]
- Schöls L, Bauer P, Schmidt T, et al. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol 2004;3:291-304. [PubMed]
- Sailer A, Scholz SW, Gibbs JR, et al. Exome sequencing in an SCA14 family demonstrates its utility in diagnosing heterogeneous diseases. Neurology 2012;79:127-31. [PubMed]
- Bras J, Guerreiro R, Hardy J. Use of next-generation sequencing and other whole-genome strategies to dissect neurological disease. Nat Rev Neurosci 2012;13:453-64. [PubMed]
- Seyhan AA. RNAi: a potential new class of therapeutic for human genetic disease. Hum Genet 2011;130:583-605. [PubMed]
- Fiszer A, Krzyzosiak WJ. Oligonucleotide-based strategies to combat polyglutamine diseases. Nucleic Acids Res 2014;42:6787-810. [PubMed]
- Fiszer A, Olejniczak M, Switonski PM, et al. An evaluation of oligonucleotide-based therapeutic strategies for polyQ diseases. BMC Mol Biol 2012;13:6. [PubMed]
- Xia H, Mao Q, Eliason SL, et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med 2004;10:816-20. [PubMed]
- Keiser MS, Geoghegan JC, Boudreau RL, et al. RNAi or overexpression: alternative therapies for Spinocerebellar Ataxia Type 1. Neurobiol Dis 2013;56:6-13. [PubMed]
- Keiser MS, Boudreau RL, Davidson BL. Broad therapeutic benefit after RNAi expression vector delivery to deep cerebellar nuclei: implications for spinocerebellar ataxia type 1 therapy. Mol Ther 2014;22:588-95. [PubMed]
- Ramachandran PS, Boudreau RL, Schaefer KA, et al. Nonallele specific silencing of ataxin-7 improves disease phenotypes in a mouse model of SCA7. Mol Ther 2014;22:1635-42. [PubMed]
- Scholefield J, Watson L, Smith D, et al. Allele-specific silencing of mutant Ataxin-7 in SCA7 patient-derived fibroblasts. Eur J Hum Genet 2014;22:1369-75. [PubMed]
- Costa Mdo C, Luna-Cancalon K, Fischer S, et al. Toward RNAi therapy for the polyglutamine disease Machado-Joseph disease. Mol Ther 2013;21:1898-908. [PubMed]
- Evers MM, Tran HD, Zalachoras I, et al. Ataxin-3 protein modification as a treatment strategy for spinocerebellar ataxia type 3: removal of the CAG containing exon. Neurobiol Dis 2013;58:49-56. [PubMed]
- Evers MM, Pepers BA, van Deutekom JC, et al. Targeting several CAG expansion diseases by a single antisense oligonucleotide. PLoS One 2011;6:e24308. [PubMed]
- Hu J, Matsui M, Gagnon KT, et al. Allele-specific silencing of mutant huntingtin and ataxin-3 genes by targeting expanded CAG repeats in mRNAs. Nat Biotechnol 2009;27:478-84. [PubMed]
- Williams AJ, Paulson HL. Polyglutarnine neurodegeneration: protein misfolding revisited. Trends Neurosci 2008;31:521-8. [PubMed]
- Zoghbi HY, Orr HT. Glutamine repeats and neurodegeneration. Annu Rev Neurosci 2000;23:217-47. [PubMed]
- Slow EJ, Graham RK, Osmand AP, et al. Absence of behavioral abnormalities and neurodegeneration in vivo despite widespread neuronal huntingtin inclusions. Proc Natl Acad Sci U S A 2005;102:11402-7. [PubMed]
- Rüb U, de Vos RA, Brunt ER, et al. Spinocerebellar ataxia type 3 (SCA3): thalamic neurodegeneration occurs independently from thalamic ataxin-3 immunopositive neuronal intranuclear inclusions. Brain Pathol 2006;16:218-27. [PubMed]
- Arrasate M, Mitra S, Schweitzer ES, et al. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 2004;431:805-10. [PubMed]
- Takahashi T, Katada S, Onodera O. Polyglutamine diseases: where does toxicity come from? what is toxicity? where are we going? J Mol Cell Biol 2010;2:180-91. [PubMed]
- Kitamura A, Kubota H. Amyloid oligomers: dynamics and toxicity in the cytosol and nucleus. FEBS J 2010;277:1369-79. [PubMed]
- Pratt WB, Gestwicki JE, Osawa Y, et al. Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annu Rev Pharmacol Toxicol 2015;55:353-71. [PubMed]
- Tsai HF, Lin SJ, Li C, et al. Decreased expression of Hsp27 and Hsp70 in transformed lymphoblastoid cells from patients with spinocerebellar ataxia type 7. Biochem Biophys Res Commun 2005;334:1279-86. [PubMed]
- Zijlstra MP, Rujano MA, Van Waarde MA, et al. Levels of DNAJB family members (HSP40) correlate with disease onset in patients with spinocerebellar ataxia type 3. Eur J Neurosci 2010;32:760-70. [PubMed]
- Ogawa K, Seki T, Onji T, et al. Mutant gammaPKC that causes spinocerebellar ataxia type 14 upregulates Hsp70, which protects cells from the mutant's cytotoxicity. Biochem Biophys Res Commun 2013;440:25-30. [PubMed]
- Fujikake N, Nagai Y, Popiel HA, et al. Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. J Biol Chem 2008;283:26188-97. [PubMed]
- Cummings CJ, Sun Y, Opal P, et al. Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum Mol Genet 2001;10:1511-8. [PubMed]
- Yang S, Huang S, Gaertig MA, et al. Age-dependent decrease in chaperone activity impairs MANF expression, leading to Purkinje cell degeneration in inducible SCA17 mice. Neuron 2014;81:349-65. [PubMed]
- Parfitt DA, Michael GJ, Vermeulen EG, et al. The ataxia protein sacsin is a functional co-chaperone that protects against polyglutamine-expanded ataxin-1. Hum Mol Genet 2009;18:1556-65. [PubMed]
- Li L, Saegusa H, Tanabe T. Deficit of heat shock transcription factor 1-heat shock 70 kDa protein 1A axis determines the cell death vulnerability in a model of spinocerebellar ataxia type 6. Genes Cells 2009;14:1253-69. [PubMed]
- Huang S, Ling JJ, Yang S, et al. Neuronal expression of TATA box-binding protein containing expanded polyglutamine in knock-in mice reduces chaperone protein response by impairing the function of nuclear factor-Y transcription factor. Brain 2011;134:1943-58. [PubMed]
- Waza M, Adachi H, Katsuno M, et al. 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat Med 2005;11:1088-95. [PubMed]
- Wang AM, Miyata Y, Klinedinst S, et al. Activation of Hsp70 reduces neurotoxicity by promoting polyglutamine protein degradation. Nat Chem Biol 2013;9:112-8. [PubMed]
- Friedman MJ, Li S, Li XJ. Activation of gene transcription by heat shock protein 27 may contribute to its neuronal protection. J Biol Chem 2009;284:27944-51. [PubMed]
- Rolfs A, Koeppen AH, Bauer I, et al. Clinical features and neuropathology of autosomal dominant spinocerebellar ataxia (SCA17). Ann Neurol 2003;54:367-75. [PubMed]
- Koide R, Kobayashi S, Shimohata T, et al. A neurological disease caused by an expanded CAG trinucleotide repeat in the TATA-binding protein gene: a new polyglutamine disease? Hum Mol Genet 1999;8:2047-53. [PubMed]
- Friedman MJ, Shah AG, Fang ZH, et al. Polyglutamine domain modulates the TBP-TFIIB interaction: implications for its normal function and neurodegeneration. Nat Neurosci 2007;10:1519-28. [PubMed]
- Friedman MJ, Wang CE, Li XJ, et al. Polyglutamine expansion reduces the association of TATA-binding protein with DNA and induces DNA binding-independent neurotoxicity. J Biol Chem 2008;283:8283-90. [PubMed]
- Shah AG, Friedman MJ, Huang S, et al. Transcriptional dysregulation of TrkA associates with neurodegeneration in spinocerebellar ataxia type 17. Hum Mol Genet 2009;18:4141-52. [PubMed]
- Mohan RD, Abmayr SM, Workman JL. Pulling complexes out of complex diseases: Spinocerebellar Ataxia 7. Rare Dis 2014;2:e28859. [PubMed]
- Chou AH, Chen CY, Chen SY, et al. Polyglutamine-expanded ataxin-7 causes cerebellar dysfunction by inducing transcriptional dysregulation. Neurochem Int 2010;56:329-39. [PubMed]
- Lim J, Crespo-Barreto J, Jafar-Nejad P, et al. Opposing effects of polyglutamine expansion on native protein complexes contribute to SCA1. Nature 2008;452:713-8. [PubMed]
- Orr HT. Cell biology of spinocerebellar ataxia. J Cell Biol 2012;197:167-77. [PubMed]
- Fryer JD, Yu P, Kang H, et al. Exercise and genetic rescue of SCA1 via the transcriptional repressor Capicua. Science 2011;334:690-3. [PubMed]
- Lam YC, Bowman AB, Jafar-Nejad P, et al. ATAXIN-1 interacts with the repressor Capicua in its native complex to cause SCA1 neuropathology. Cell 2006;127:1335-47. [PubMed]
- Chou AH, Chen SY, Yeh TH, et al. HDAC inhibitor sodium butyrate reverses transcriptional downregulation and ameliorates ataxic symptoms in a transgenic mouse model of SCA3. Neurobiol Dis 2011;41:481-8. [PubMed]
- Duncan CE, An MC, Papanikolaou T, et al. Histone deacetylase-3 interacts with ataxin-7 and is altered in a spinocerebellar ataxia type 7 mouse model. Mol Neurodegener 2013;8:42. [PubMed]
- Venkatraman A, Hu YS, Didonna A, et al. The histone deacetylase HDAC3 is essential for Purkinje cell function, potentially complicating the use of HDAC inhibitors in SCA1. Hum Mol Genet 2014;23:3733-45. [PubMed]
- Galka-Marciniak P, Urbanek MO, Krzyzosiak WJ. Triplet repeats in transcripts: structural insights into RNA toxicity. Biol Chem 2012;393:1299-315. [PubMed]
- Todd PK, Paulson HL. RNA-mediated neurodegeneration in repeat expansion disorders. Ann Neurol 2010;67:291-300. [PubMed]
- Li LB, Yu Z, Teng X, et al. RNA toxicity is a component of ataxin-3 degeneration in Drosophila. Nature 2008;453:1107-11. [PubMed]
- White MC, Gao R, Xu W, et al. Inactivation of hnRNP K by expanded intronic AUUCU repeat induces apoptosis via translocation of PKCdelta to mitochondria in spinocerebellar ataxia 10. PLoS Genet 2010;6:e1000984. [PubMed]
- Sato N, Amino T, Kobayashi K, et al. Spinocerebellar ataxia type 31 is associated with "inserted" penta-nucleotide repeats containing (TGGAA)n. Am J Hum Genet 2009;85:544-57. [PubMed]
- Kearse MG, Todd PK. Repeat-associated non-AUG translation and its impact in neurodegenerative disease. Neurotherapeutics 2014;11:721-31. [PubMed]
- Zu T, Gibbens B, Doty NS, et al. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci U S A 2011;108:260-5. [PubMed]
- Koob MD, Moseley ML, Schut LJ, et al. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). Nat Genet 1999;21:379-84. [PubMed]
- Daughters RS, Tuttle DL, Gao W, et al. RNA gain-of-function in spinocerebellar ataxia type 8. PLoS Genet 2009;5:e1000600. [PubMed]
- Moseley ML, Zu T, Ikeda Y, et al. Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat Genet 2006;38:758-69. [PubMed]
- Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev 2011;91:827-87. [PubMed]
- Schaefer A, O'Carroll D, Tan CL, et al. Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med 2007;204:1553-8. [PubMed]
- Damiani D, Alexander JJ, O'Rourke JR, et al. Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina. J Neurosci 2008;28:4878-87. [PubMed]
- Lee Y, Samaco RC, Gatchel JR, et al. miR-19, miR-101 and miR-130 co-regulate ATXN1 levels to potentially modulate SCA1 pathogenesis. Nat Neurosci 2008;11:1137-9. [PubMed]
- Tan JY, Vance KW, Varela MA, et al. Cross-talking noncoding RNAs contribute to cell-specific neurodegeneration in SCA7. Nat Struct Mol Biol 2014;21:955-61. [PubMed]
- McCann C, Holohan EE, Das S, et al. The Ataxin-2 protein is required for microRNA function and synapse-specific long-term olfactory habituation. Proc Natl Acad Sci U S A 2011;108:E655-62. [PubMed]
- van de Leemput J, Chandran J, Knight MA, et al. Deletion at ITPR1 underlies ataxia in mice and spinocerebellar ataxia 15 in humans. PLoS Genet 2007;3:e108. [PubMed]
- Iwaki A, Kawano Y, Miura S, et al. Heterozygous deletion of ITPR1, but not SUMF1, in spinocerebellar ataxia type 16. J Med Genet 2008;45:32-5. [PubMed]
- Shuvaev AN, Horiuchi H, Seki T, et al. Mutant PKCgamma in spinocerebellar ataxia type 14 disrupts synapse elimination and long-term depression in Purkinje cells in vivo. J Neurosci 2011;31:14324-34. [PubMed]
- Adachi N, Kobayashi T, Takahashi H, et al. Enzymological analysis of mutant protein kinase Cgamma causing spinocerebellar ataxia type 14 and dysfunction in Ca2+ homeostasis. J Biol Chem 2008;283:19854-63. [PubMed]
- Zhuchenko O, Bailey J, Bonnen P, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet 1997;15:62-9. [PubMed]
- Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 1996;87:543-52. [PubMed]
- Chopra R, Shakkottai VG. The role for alterations in neuronal activity in the pathogenesis of polyglutamine repeat disorders. Neurotherapeutics 2014;11:751-63. [PubMed]
- Lin X, Antalffy B, Kang D, et al. Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nat Neurosci 2000;3:157-63. [PubMed]
- Serra HG, Byam CE, Lande JD, et al. Gene profiling links SCA1 pathophysiology to glutamate signaling in Purkinje cells of transgenic mice. Hum Mol Genet 2004;13:2535-43. [PubMed]
- Vig PJ, Wei J, Shao Q, et al. Suppression of calbindin-D28k expression exacerbates SCA1 phenotype in a disease mouse model. Cerebellum 2012;11:718-32. [PubMed]
- Vig PJ, Hearst SM, Shao Q, et al. Knockdown of acid-sensing ion channel 1a (ASIC1a) suppresses disease phenotype in SCA1 mouse model. Cerebellum 2014;13:479-90. [PubMed]
- Hansen ST, Meera P, Otis TS, et al. Changes in Purkinje cell firing and gene expression precede behavioral pathology in a mouse model of SCA2. Hum Mol Genet 2013;22:271-83. [PubMed]
- Liu J, Tang TS, Tu H, et al. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci 2009;29:9148-62. [PubMed]
- Chen X, Tang TS, Tu H, et al. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J Neurosci 2008;28:12713-24. [PubMed]
- Kasumu AW, Liang X, Egorova P, et al. Chronic suppression of inositol 1,4,5-triphosphate receptor-mediated calcium signaling in cerebellar purkinje cells alleviates pathological phenotype in spinocerebellar ataxia 2 mice. J Neurosci 2012;32:12786-96. [PubMed]
- Gao Z, van Beugen BJ, De Zeeuw CI. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci 2012;13:619-35. [PubMed]
- Takayasu Y, Iino M, Takatsuru Y, et al. Functions of glutamate transporters in cerebellar Purkinje cell synapses. Acta Physiol (Oxf) 2009;197:1-12. [PubMed]
- Ikeda Y, Dick KA, Weatherspoon MR, et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet 2006;38:184-90. [PubMed]
- Inoue T, Lin X, Kohlmeier KA, et al. Calcium dynamics and electrophysiological properties of cerebellar Purkinje cells in SCA1 transgenic mice. J Neurophysiol 2001;85:1750-60. [PubMed]
- Custer SK, Garden GA, Gill N, et al. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat Neurosci 2006;9:1302-11. [PubMed]
- Garden GA, Libby RT, Fu YH, et al. Polyglutamine-expanded ataxin-7 promotes non-cell-autonomous purkinje cell degeneration and displays proteolytic cleavage in ataxic transgenic mice. J Neurosci 2002;22:4897-905. [PubMed]
- Chopra R, Shakkottai VG. Translating cerebellar Purkinje neuron physiology to progress in dominantly inherited ataxia. Future Neurol 2014;9:187-96. [PubMed]
- Figueroa KP, Minassian NA, Stevanin G, et al. KCNC3: phenotype, mutations, channel biophysics-a study of 260 familial ataxia patients. Hum Mutat 2010;31:191-6. [PubMed]
- Waters MF, Minassian NA, Stevanin G, et al. Mutations in voltage-gated potassium channel KCNC3 cause degenerative and developmental central nervous system phenotypes. Nat Genet 2006;38:447-51. [PubMed]
- Duarri A, Jezierska J, Fokkens M, et al. Mutations in potassium channel kcnd3 cause spinocerebellar ataxia type 19. Ann Neurol 2012;72:870-80. [PubMed]
- Lee YC, Durr A, Majczenko K, et al. Mutations in KCND3 cause spinocerebellar ataxia type 22. Ann Neurol 2012;72:859-69. [PubMed]
- Walter JT, Alvina K, Womack MD, et al. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci 2006;9:389-97. [PubMed]
- Lorenzon NM, Lutz CM, Frankel WN, et al. Altered calcium channel currents in Purkinje cells of the neurological mutant mouse leaner. J Neurosci 1998;18:4482-9. [PubMed]
- Dove LS, Abbott LC, Griffith WH. Whole-cell and single-channel analysis of P-type calcium currents in cerebellar Purkinje cells of leaner mutant mice. J Neurosci 1998;18:7687-99. [PubMed]
- Wakamori M, Yamazaki K, Matsunodaira H, et al. Single tottering mutations responsible for the neuropathic phenotype of the P-type calcium channel. J Biol Chem 1998;273:34857-67. [PubMed]
- Alviña K, Khodakhah K. KCa channels as therapeutic targets in episodic ataxia type-2. J Neurosci 2010;30:7249-57. [PubMed]
- Hourez R, Servais L, Orduz D, et al. Aminopyridines correct early dysfunction and delay neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J Neurosci 2011;31:11795-807. [PubMed]
- Dell'Orco JM, Wasserman AH, Chopra R, et al. Neuronal Atrophy Early in Degenerative Ataxia Is a Compensatory Mechanism to Regulate Membrane Excitability. J Neurosci 2015;35:11292-307. [PubMed]
- Watase K, Barrett CF, Miyazaki T, et al. Spinocerebellar ataxia type 6 knockin mice develop a progressive neuronal dysfunction with age-dependent accumulation of mutant CaV2.1 channels. Proc Natl Acad Sci U S A 2008;105:11987-92. [PubMed]
- Perkins EM, Clarkson YL, Sabatier N, et al. Loss of beta-III spectrin leads to Purkinje cell dysfunction recapitulating the behavior and neuropathology of spinocerebellar ataxia type 5 in humans. J Neurosci 2010;30:4857-67. [PubMed]
- Kasumu AW, Hougaard C, Rode F, et al. Selective positive modulator of calcium-activated potassium channels exerts beneficial effects in a mouse model of spinocerebellar ataxia type 2. Chem Biol 2012;19:1340-53. [PubMed]
- Shakkottai VG, do Carmo Costa M, Dell'Orco JM, et al. Early changes in cerebellar physiology accompany motor dysfunction in the polyglutamine disease spinocerebellar ataxia type 3. J Neurosci 2011;31:13002-14. [PubMed]
- Gao Z, Todorov B, Barrett CF, et al. Cerebellar ataxia by enhanced Ca(V)2.1 currents is alleviated by Ca2+-dependent K+-channel activators in Cacna1a(S218L) mutant mice. J Neurosci 2012;32:15533-46. [PubMed]
- Ristori G, Romano S, Visconti A, et al. Riluzole in cerebellar ataxia: a randomized, double-blind, placebo-controlled pilot trial. Neurology 2010;74:839-45. [PubMed]
- Romano S, Coarelli G, Marcotulli C, et al. Riluzole in patients with hereditary cerebellar ataxia: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2015;14:985-91. [PubMed]
- Cao YJ, Dreixler JC, Couey JJ, et al. Modulation of recombinant and native neuronal SK channels by the neuroprotective drug riluzole. Eur J Pharmacol 2002;449:47-54. [PubMed]
- Lam J, Coleman N, Garing AL, et al. The therapeutic potential of small-conductance KCa2 channels in neurodegenerative and psychiatric diseases. Expert Opin Ther Targets 2013;17:1203-20. [PubMed]
- Jacobi H, Reetz K, du Montcel ST, et al. Biological and clinical characteristics of individuals at risk for spinocerebellar ataxia types 1, 2, 3, and 6 in the longitudinal RISCA study: analysis of baseline data. Lancet Neurol 2013;12:650-8. [PubMed]


