Interfering with the IFN-γ/CXCL10 pathway to develop new targeted treatments for vitiligo
Vitiligo is a common, disfiguring autoimmune disease caused by the destruction of epidermal melanocytes. It presents with patchy depigmentation of skin, which significantly affects patients’ self-esteem and quality of life. The mainstay of vitiligo treatment is topical steroids, calcineurin inhibitors, and/or narrow band UVB (nbUVB) phototherapy (1). These treatments utilize a non-targeted approach with moderate efficacy, but are used off-label, as they are not Food and Drug Administration (FDA)-approved for use in vitiligo. Currently, the only FDA-approved treatment for the disease is monobenzone cream (Benoquin®), which is actually used to permanently depigment, rather than repigment, the skin. This treatment results in an even skin tone, and can be appropriate for patients with extensive disease (2,3). However, it is one of a limited number of treatments used in medicine to intentionally make disease worse, and treatments focused on reversing vitiligo, with better efficacy, are greatly needed.
Over the past three decades, researchers have made significant progress in developing new, more targeted treatments for autoimmune diseases. Unlike the majority of traditional drugs, biologics and other targeted therapies are designed to selectively inhibit specific components of an inflammatory pathway responsible for driving disease pathogenesis. This approach has the potential to revolutionize the management of many inflammatory diseases, including skin diseases like psoriasis, urticaria, and atopic dermatitis (4-6). Tumor necrosis factor (TNF)-α inhibitors, for example, initially developed for the treatment of rheumatoid arthritis (RA), were later found to significantly improve psoriasis skin and joint inflammation, suggesting that TNF-α is a key inflammatory mediator in disease pathogenesis (7).
TNF-α is also mildly elevated in the lesional skin and blood of patients with vitiligo (8-11), and so there was initial excitement about testing existing biologic therapies that target TNF-α for vitiligo patients. However, multiple small trials to test these drugs in vitiligo failed, and in fact induced or worsened disease in several patients (12-16). A recent review attempted to compile these studies and argue that TNF-α inhibitors, while ineffective at repigmentation, may have stabilized disease (17). However the studies did not track disease progression, and were not powered or conducted long enough to measure stabilization (13,14,16). In fact, one study claimed stabilization in a patient, but the figure revealed progression (13), and one of us (JEH) has cared for a patient with progressive disease that continued to progress unimpeded during treatment with etanercept for psoriasis. In summary, it appears that TNF-α inhibitors are ineffective for vitiligo, which suggests that vitiligo is driven by a distinct inflammatory pathway that is not shared with psoriasis.
Thus, for the development of new, targeted therapies for vitiligo the autoimmune pathways responsible for progression would have to be determined. We found that gene expression profiling in lesional skin of patients and a mouse model of vitiligo indicated an increase in expression of IFN-γ and IFN-γ induced genes (18,19). In this context, vitiligo is more similar to alopecia areata (AA), an autoimmune disease that presents with patchy hair loss, than it is to psoriasis (20). We also found that CXCL10, an IFN-γ induced chemokine, is elevated in serum of patients with vitiligo, and that CXCR3, its cognate receptor, was upregulated on autoreactive T cells in the blood and skin of patients with vitiligo. We then demonstrated that the IFN-γ/CXCL10 axis is functionally required for both progression and maintenance of the disease in a mouse model, and therefore can be therapeutically targeted to reverse depigmentation (18,19).
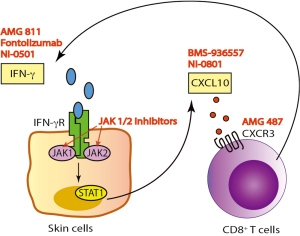
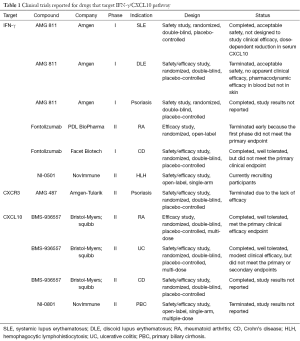
IFN-γ/CXCL10 signaling begins with binding of IFN-γ to the IFN-γ heterodimeric receptor that activates the Janus Kinase (JAK)-STAT pathway, which leads to STAT1 activation. This is followed by STAT1 translocation to the nucleus and subsequent binding to the promoter region of immediate-early IFNγ-inducible genes, resulting in CXCL10 transcription (Figure 1) (21). A number of human monoclonal antibodies against IFN-γ have been tested in clinical trials in patients with psoriasis, Crohn’s disease (CD), and systemic lupus erythematosus (SLE) (Table 1) (22-24). Although treatment showed modulation of the gene expression associated with IFN-γ signaling, these trials failed to meet the primary endpoint of the study. This is likely because IFN-γ is not driving these diseases, but in contrast, existing data strongly implicate IFN-γ as the critical driver of autoimmunity in vitiligo, and support testing IFN-γ antibodies in this patient population.
Full table
JAKs comprise a family of intracellular, non-receptor tyrosine kinases that transduce cytokine-mediated signals to activate the STAT1 transcription factor. Members include JAK1, JAK2, JAK3, and TYK2. JAK1 and JAK2 are directly involved in IFN-γ signal transduction following its binding to the receptor (25), and are thus downstream signaling mediators that could be appropriate targets for vitiligo therapy. Indeed, two JAK inhibitors, tofacitinib (pan-JAK inhibitor, approved for RA) and ruxolitinib (JAK1/2 inhibitor, approved for myelofibrosis and polycythemia vera), were each recently reported to induce substantial repigmentation in two separate vitiligo patients (26,27). Our patient treated with ruxolitinib demonstrated a decrease in his serum CXCL10 level shortly after initiating treatment, supporting its role as an IFN-γ signaling inhibitor (27).
Using the same reasoning, we also hypothesized that STAT1 inhibitors could potentially treat vitiligo. Based on a study that reported that statins, or HMG-CoA reductase inhibitors, could block STAT1 function in vitro (28) and a case report in which a patient improved significantly after taking simvastatin (29), we tested simvastatin as a treatment in our mouse model. It was effective at both inhibiting and reversing vitiligo in the model although its mechanism of action was not clear, as it appeared to have multiple pleotropic effects on T cells during the progression of disease (30). Studies are ongoing to test its efficacy in vitiligo patients (31).
Another treatment strategy for vitiligo would be to directly target CXCL10 or its receptor CXCR3. This could possibly be a safer approach, as it interrupts the disease process further downstream without interfering with the other effectors of IFN-γ. We found that this was highly effective in preventing and reversing vitiligo in our mouse model (19). Two separate human anti-CXCL10 monoclonal antibodies have been tested in phase 2 clinical trials in patients with RA and ulcerative colitis (UC). While the treatment was well tolerated, it only showed moderate clinical efficacy (Table 1) (32,33). In addition, several classes of CXCR3 small molecule inhibitors have been described, yet despite promising results in preclinical studies, only one has been progressed to a phase 2 clinical trial (34). This was to evaluate the safety and efficacy of AMG-487 for the treatment of patients with psoriasis, and was terminated early due to lack of efficacy. This is likely due to the fact that psoriasis is driven by cell types other than CXCR3-expressing T cells (35). However, these cells appear to be the main effectors in vitiligo (19), and therefore vitiligo may be the optimal inflammatory disease to test these CXCR3 antagonists.
The use of targeted immunotherapy has revolutionized our management of patients with psoriasis, RA, and inflammatory bowel disease, and understanding the key signaling pathways that drive the pathogenesis of each disease is a critical step for the development of these treatments. Taken together, recent discoveries suggest that targeting the IFN-γ-CXCL10-CXCR3 axis has excellent potential for developing new vitiligo treatments.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet 2015;386:74-84. [PubMed]
- AlGhamdi KM, Kumar A. Depigmentation therapies for normal skin in vitiligo universalis. J Eur Acad Dermatol Venereol 2011;25:749-57. [PubMed]
- Mosher DB, Parrish JA, Fitzpatrick TB. Monobenzylether of hydroquinone. A retrospective study of treatment of 18 vitiligo patients and a review of the literature. Br J Dermatol 1977;97:669-79. [PubMed]
- Hsu S, Papp KA, Lebwohl MG, et al. Consensus guidelines for the management of plaque psoriasis. Arch Dermatol 2012;148:95-102. [PubMed]
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med 2014;371:130-9. [PubMed]
- Maurer M, Rosén K, Hsieh HJ, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med 2013;368:924-35. [PubMed]
- Leonardi CL, Romiti R, Tebbey PW. Ten years on: the impact of biologics on the practice of dermatology. Dermatol Clin 2015;33:111-25. [PubMed]
- Grimes PE, Morris R, Avaniss-Aghajani E, et al. Topical tacrolimus therapy for vitiligo: therapeutic responses and skin messenger RNA expression of proinflammatory cytokines. J Am Acad Dermatol 2004;51:52-61. [PubMed]
- Birol A, Kisa U, Kurtipek GS, et al. Increased tumor necrosis factor alpha (TNF-alpha) and interleukin 1 alpha (IL1-alpha) levels in the lesional skin of patients with nonsegmental vitiligo. Int J Dermatol 2006;45:992-3. [PubMed]
- Seif El Nasr H, Shaker OG, Fawzi MM, et al. Basic fibroblast growth factor and tumour necrosis factor alpha in vitiligo and other hypopigmented disorders: suggestive possible therapeutic targets. J Eur Acad Dermatol Venereol 2013;27:103-8. [PubMed]
- van den Boorn JG, Konijnenberg D, Dellemijn TA, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol 2009;129:2220-32. [PubMed]
- Speeckaert R, Speeckaert MM, van Geel N. Why treatments do(n't) work in vitiligo: An autoinflammatory perspective. Autoimmun Rev 2015;14:332-40. [PubMed]
- Alghamdi KM, Khurrum H, Taieb A, et al. Treatment of generalized vitiligo with anti-TNF-α Agents. J Drugs Dermatol 2012;11:534-9. [PubMed]
- Kim NH, Torchia D, Rouhani P, et al. Tumor necrosis factor-α in vitiligo: direct correlation between tissue levels and clinical parameters. Cutan Ocul Toxicol 2011;30:225-7. [PubMed]
- Alghamdi KM, Khurrum H, Rikabi A. Worsening of vitiligo and onset of new psoriasiform dermatitis following treatment with infliximab. J Cutan Med Surg 2011;15:280-4. [PubMed]
- Rigopoulos D, Gregoriou S, Larios G, et al. Etanercept in the treatment of vitiligo. Dermatology 2007;215:84-5. [PubMed]
- Webb KC, Tung R, Winterfield LS, et al. Tumour necrosis factor-α inhibition can stabilize disease in progressive vitiligo. Br J Dermatol 2015;173:641-50. [PubMed]
- Harris JE, Harris TH, Weninger W, et al. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J Invest Dermatol 2012;132:1869-76. [PubMed]
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med 2014;6:223ra23.
- Harris JE. Vitiligo and alopecia areata: apples and oranges? Exp Dermatol 2013;22:785-9. [PubMed]
- Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol 1997;15:563-91. [PubMed]
- Chen P, Vu T, Narayanan A, et al. Pharmacokinetic and pharmacodynamic relationship of AMG 811, an anti-IFN-γ IgG1 monoclonal antibody, in patients with systemic lupus erythematosus. Pharm Res 2015;32:640-53. [PubMed]
- Harden JL, Johnson-Huang LM, Chamian MF, et al. Humanized anti-IFN-γ (HuZAF) in the treatment of psoriasis. J Allergy Clin Immunol 2015;135:553-6. [PubMed]
- Reinisch W, de Villiers W, Bene L, et al. Fontolizumab in moderate to severe Crohn's disease: a phase 2, randomized, double-blind, placebo-controlled, multiple-dose study. Inflamm Bowel Dis 2010;16:233-42. [PubMed]
- Rodig SJ, Meraz MA, White JM, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 1998;93:373-83. [PubMed]
- Craiglow BG, King BA. Tofacitinib Citrate for the Treatment of Vitiligo: A Pathogenesis-Directed Therapy. JAMA Dermatol 2015;151:1110-2. [PubMed]
- Harris JE, Rashighi M, Nguyen N, et al. apid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata. J Am Acad Dermatol 2015. Available online: http://www.jaad.org/article/S0190-9622(15)02400-7/fulltext
- Zhao Y, Gartner U, Smith FJ, et al. Statins downregulate K6a promoter activity: a possible therapeutic avenue for pachyonychia congenita. J Invest Dermatol 2011;131:1045-52. [PubMed]
- Noël M, Gagné C, Bergeron J, et al. Positive pleiotropic effects of HMG-CoA reductase inhibitor on vitiligo. Lipids Health Dis 2004;3:7. [PubMed]
- Agarwal P, Rashighi M, Essien KI, et al. Simvastatin prevents and reverses depigmentation in a mouse model of vitiligo. J Invest Dermatol 2015;135:1080-8. [PubMed]
- Clinical Trial of Simvastatin to Treat Generalized Vitiligo. Available online: https://clinicaltrials.gov/ct2/show/NCT01517893
- Mayer L, Sandborn WJ, Stepanov Y, et al. Anti-IP-10 antibody (BMS-936557) for ulcerative colitis: a phase II randomised study. Gut 2014;63:442-50. [PubMed]
- Yellin M, Paliienko I, Balanescu A, et al. A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum 2012;64:1730-9. [PubMed]
- Jenh CH, Cox MA, Cui L, et al. A selective and potent CXCR3 antagonist SCH 546738 attenuates the development of autoimmune diseases and delays graft rejection. BMC Immunol 2012;13:2. [PubMed]
- Horuk R. Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov 2009;8:23-33. [PubMed]


