Integr(at)in(g) EGFR therapy in HNSCC
Patients with locally advanced head and neck squamous cell carcinomas (HNSCCs) are usually addressed to surgery and/or radiotherapy. The addition of chemotherapy to radiotherapy has also been extensively investigated, but treatment outcome often remained disappointing (1). Based on high levels of epidermal growth factor (EGFR) expression detected in approximately 90% of HNSCC, and associated to worse clinical outcome and decreased response to radiotherapy (2), the anti-EGFR monoclonal antibody cetuximab has been approved for treatment of patients with HNSCC (1). A paper recently published in Journal of National Cancer Institute by Eke and colleagues (3) demonstrated that simultaneous targeting of β1 integrin and EGFR is a promising approach to overcome radioresistance in preclinical HNSCC models. Mechanistically, radioresistance depends on pro-survival signalling transduced by a protein complex of focal adhesion kinase (FAK) and extracellular signal-regulated kinase (ERK1): combined β1 integrin/EGFR blocking is able to interfere with these signals.
Integrins are heterodimeric cell-surface molecules (formed by α and β subunits) that mediate cell-matrix interactions. In addition, even if not provided with intrinsic kinase activity, integrins mediate from the extracellular space into the cell through adaptor molecules such as FAK, p130Cas, Src-family kinases and GTPases of the Rho family (4,5). Via these molecules, integrin cooperatively interacts with receptor tyrosine kinase (RTK) to regulate cell survival, proliferation, adhesion, and migration (6). In HNSCC, β1 integrin overexpression has been found and related to tumour therapy resistance (7,8).
Eke and colleagues (3) reported that β1 integrin inhibition, by either the antibody AIIB2 or silencing with β1 integrin siRNA, activates EGFR associated signalling in HNSCCs. Particularly, the authors observed an increase of ERK1/2 phosphorylation and a dissociation of the FAK-ERK1 protein complex, both in vitro and in vivo (Figure 1). It is known that the Ras/Raf/MEK/ERK pathway is one of the signalling pathways activated downstream to EGFR (9) as well as to integrins (10). Similarly, FAK transduces signal from β1 integrins and EGFR (11) through autophosphorylation of tyrosine 397 (12), thus inducing cell motility, proliferation, and the stress response to ionizing radiation and chemotherapy (13-15). Shibue et al. (16) showed that β1 integrin-FAK signalling directs the proliferation of metastatic cancer cells disseminated in the lungs: β1 integrins regulate FAK activation in these metastatic cells, and inhibition of both proteins reduces cell proliferation (16).
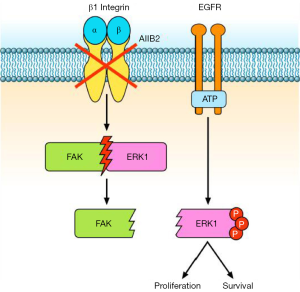
A relationship between EGFR and β1 integrin pathway has been also demonstrated in lung cancer. Morello et al. (17) reported that β1 integrin controls EGFR signalling and tumorigenic properties of lung cancer cells. Ju et al. (18) showed that β1 integrin over-expression is associated with acquired resistance to tyrosine kinase inhibitor gefitinib in non-small cell lung cancer (NSCLC), accompanied with increase of the cells’ adhesion and migration. Moreover, the sensitivity of NSCLC cells to gefitinib is negatively correlated with levels of β1 integrin protein expression (18). In another study (19), the integrin β1/Src/Akt signalling pathway has been identified as a key mediator of acquired resistance to erlotinib in lung cancer: gene silencing of β1 integrin restored sensitivity to erlotinib and reduced Src and Akt phosphorylation/activation after erlotinib treatment. In tumour samples from patients with lung cancer refractory to erlotinib and/or gefitinib, increased expression of integrin β1, α5, and/or α2 was also observed (19). Other studies reported that EGFR inhibition is related to different negative feedback loops involving MEK1/2 and other bypass signalling, often mediated by β1 integrin (20). Conversely, the work by Eke et al. (3) demonstrated that β1 integrin inhibition induces EGFR activation, with consequent overactivation of components of the Ras pathway. Based on these data, Eke and colleagues (3) tested the combination of β1 integrin inhibition by AIIB2 and EGFR inhibition by cetuximab in HNSCC models. They found that the combined treatment is more effective than single agents in inducing cytotoxicity and radiosensitization of HNSCC cell lines (Figure 2). In tumour xenografts, the combination AIIB2/cetuximab/radiotherapy produced higher tumour control rates compared to single anti-β1 integrin treatment. On the other hand, in a different tumour model, Poschau and colleagues demonstrated that both β1 integrin and EGFR targeting are inefficient to radiochemosensitize colorectal cancer cells (21).
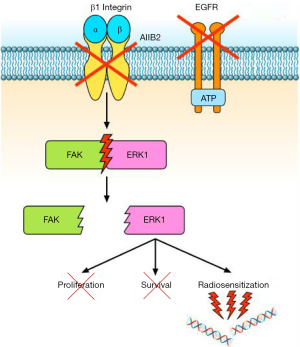
Ionizing radiations are able to induce damages to several sub-cellular structures, from the plasma membrane to the cell nucleus. Particularly, in cancer therapy, radiation-induced cytotoxicity is closely linked to DNA damage (22). In this respect, several studies report the involvement of nuclear EGFR in DNA repair, for both non-homologous end joining (via DNA-protein kinase) and homologous recombination (via Rad51) (23-25). The role of β1 integrins in this context is less known. However, several studies have reported that β1 integrin targeting enhances radiochemosensitivity in different tumour types (13,26-28). In fact, β1 integrins may regulate chromatin structure by increasing acetylation of the core histone H3 and by reducing the interaction of the linker histone H1 with DNA (29). Moreover, they have been involved in the protection from bleomycin-induced DNA breakage (30). The results obtained by Eke and colleagues (3) suggest that cooperative EGFR/β1 integrins interactions may play a critical role in DNA damage repair; therefore, the simultaneous inhibition of both signalling pathways may significantly improve radiosensitization of HNSCC models.
In the paper by Eke et al. (3), an interactome analysis on deregulated phosphoproteins, followed by network Betweeness Centrality (BC) analysis (31) revealed that simultaneous EGFR/β1 integrin inhibition induces a stronger perturbation of signalling compared to single EGFR or β1 integrin targeting. Particularly, the addition of cetuximab to AIIB2 prevents the AIIB2-induced hyperphosphorylation of Raf/MEK/ERK and FAK signalling. In different human cancer cell lines including ovarian, lung and HNSCC cells, FAK has been described downstream to Ras/Raf/MEK/ERK pathway (11,12,14,32). In order to evaluate the role of FAK downstream to β1 integrin and EGFR, as well as its interaction with the Ras pathway, the authors performed modulation (down-regulation/overexpression) of both FAK and ERK1 by siRNAs or by expression vectors. They found that FAK plays a key role in the radiosensitization of HNSCC cell lines. Moreover, the authors concluded that FAK operates downstream to ERK1, regulating the DNA damage and survival response controlled by β1 integrin and EGFR (3).
Altogether, the results by Eke demonstrate the efficacy of simultaneous β1 integrin/EGFR targeting in combination with radiotherapy in HNSCC tumours and propose this strategy as a reasonable and feasible option to overcome tumour radioresistance and diminish tumour recurrence in patients. However, the feasibility of β1 integrin targeting in cancer patients needs further evaluation. In 2014, a first-in-human clinical trial testing Fc-engineered IgG1 monoclonal antibody targeting integrin α5β1 was performed to evaluate tolerability, maximum tolerated dose, pharmacokinetics, pharmacodynamics and preliminary anti-tumour activity in patients with advanced solid tumours. Unfortunately, the trial was prematurely terminated without reaching end-points for the high toxicity (33). Moreover, since Eke and colleagues found that two out of the ten tested models do not respond to combination therapy, further studies will be required to understand the mechanisms of nonsusceptibility for β1 integrin/EGFR targeting. The knowledge of molecular determinants of response, i.e., FAK phosphorylation/dephosphorylation after exposition to AIIB2/cetuximab, could allow a selection of patients who will potentially benefit from this kind of therapy.
Acknowledgements
Funding: This study was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC) Investigator Grant 2015-2017 (IG-15388) to RB. R Rosa is supported by a post-Doctoral Fellowship from Fondazione Veronesi.
Footnote
Provenance: This is a Guest Commentary commissioned by Section Editor Hongcheng Zhu, MD, PhD (Department of Radiation Oncology, First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflict of Interest: The authors have no conflict of interest to declare.
References
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010;11:21-8. [PubMed]
- Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res 2002;62:7350-6. [PubMed]
- Eke I, Zscheppang K, Dickreuter E, et al. Simultaneous β1 integrin-EGFR targeting and radiosensitization of human head and neck cancer. J Natl Cancer Inst 2015;107:dju419. [PubMed]
- Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta 2001;1540:1-21. [PubMed]
- Welsh CF, Assoian RK. A growing role for Rho family GTPases as intermediaries in growth factor- and adhesion-dependent cell cycle progression. Biochim Biophys Acta 2000;1471:M21-9. [PubMed]
- Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim Biophys Acta 2007;1775:163-80.
- Eriksen JG, Steiniche T, Søgaard H, et al. Expression of integrins and E-cadherin in squamous cell carcinomas of the head and neck. APMIS 2004;112:560-8. [PubMed]
- Janes SM, Watt FM. New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer 2006;6:175-83. [PubMed]
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 2008;358:1160-74. [PubMed]
- Chen JC, Chen YJ, Lin CY, et al. Amphiregulin enhances alpha6beta1 integrin expression and cell motility in human chondrosarcoma cells through Ras/Raf/MEK/ERK/AP-1 pathway. Oncotarget 2015;6:11434-46. [PubMed]
- Cance WG, Kurenova E, Marlowe T, et al. Disrupting the scaffold to improve focal adhesion kinase-targeted cancer therapeutics. Sci Signal 2013;6:pe10. [PubMed]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 2006;18:516-23. [PubMed]
- Eke I, Deuse Y, Hehlgans S, et al. β1Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J Clin Invest 2012;122:1529-40. [PubMed]
- Hehlgans S, Eke I, Cordes N. Targeting FAK radiosensitizes 3-dimensional grown human HNSCC cells through reduced Akt1 and MEK1/2 signaling. Int J Radiat Oncol Biol Phys 2012;83:e669-76. [PubMed]
- Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 2014;14:598-610. [PubMed]
- Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci U S A 2009;106:10290-5. [PubMed]
- Morello V, Cabodi S, Sigismund S, et al. β1 integrin controls EGFR signaling and tumorigenic properties of lung cancer cells. Oncogene 2011;30:4087-96. [PubMed]
- Ju L, Zhou C, Li W, et al. Integrin beta1 over-expression associates with resistance to tyrosine kinase inhibitor gefitinib in non-small cell lung cancer. J Cell Biochem 2010;111:1565-74. [PubMed]
- Kanda R, Kawahara A, Watari K, et al. Erlotinib resistance in lung cancer cells mediated by integrin β1/Src/Akt-driven bypass signaling. Cancer Res 2013;73:6243-53. [PubMed]
- Eke I, Storch K, Krause M, et al. Cetuximab attenuates its cytotoxic and radiosensitizing potential by inducing fibronectin biosynthesis. Cancer Res 2013;73:5869-79. [PubMed]
- Poschau M, Dickreuter E, Singh-Müller J, et al. EGFR and β1-integrin targeting differentially affect colorectal carcinoma cell radiosensitivity and invasion. Radiother Oncol 2015;116:510-6. [PubMed]
- Raleigh DR, Haas-Kogan DA. Molecular targets and mechanisms of radiosensitization using DNA damage response pathways. Future Oncol 2013;9:219-33. [PubMed]
- Wang Y, Yuan JL, Zhang YT, et al. Inhibition of both EGFR and IGF1R sensitized prostate cancer cells to radiation by synergistic suppression of DNA homologous recombination repair. PLoS One 2013;8:e68784. [PubMed]
- Huang S, Benavente S, Armstrong EA, et al. p53 modulates acquired resistance to EGFR inhibitors and radiation. Cancer Res 2011;71:7071-9. [PubMed]
- Dittmann K, Mayer C, Fehrenbacher B, et al. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem 2005;280:31182-9. [PubMed]
- Huang C, Park CC, Hilsenbeck SG, et al. β1 integrin mediates an alternative survival pathway in breast cancer cells resistant to lapatinib. Breast Cancer Res 2011;13:R84. [PubMed]
- Estrugo D, Fischer A, Hess F, et al. Ligand bound beta1 integrins inhibit procaspase-8 for mediating cell adhesion-mediated drug and radiation resistance in human leukemia cells. PLoS One 2007;2:e269. [PubMed]
- Shields MA, Krantz SB, Bentrem DJ, et al. Interplay between β1-integrin and Rho signaling regulates differential scattering and motility of pancreatic cancer cells by snail and Slug proteins. J Biol Chem 2012;287:6218-29. [PubMed]
- Rose JL, Huang H, Wray SF, et al. Integrin engagement increases histone H3 acetylation and reduces histone H1 association with DNA in murine lung endothelial cells. Mol Pharmacol 2005;68:439-46. [PubMed]
- Rose JL, Reeves KC, Likhotvorik RI, et al. Base excision repair proteins are required for integrin-mediated suppression of bleomycin-induced DNA breakage in murine lung endothelial cells. J Pharmacol Exp Ther 2007;321:318-26. [PubMed]
- Unger K. Integrative radiation systems biology. Radiat Oncol 2014;9:21. [PubMed]
- Rea K, Sensi M, Anichini A, et al. EGFR/MEK/ERK/CDK5-dependent integrin-independent FAK phosphorylated on serine 732 contributes to microtubule depolymerization and mitosis in tumor cells. Cell Death Dis 2013;4:e815. [PubMed]
- Mateo J, Berlin J, de Bono JS, et al. A first-in-human study of the anti-α5β1 integrin monoclonal antibody PF-04605412 administered intravenously to patients with advanced solid tumors. Cancer Chemother Pharmacol 2014;74:1039-46. [PubMed]


