Role of microRNAs in chemoresistance
Introduction
A key issue in cancer therapy is the recurrent incidence of resistance to drug treatments that allows cancer cells to proliferate exponentially and to become more antagonistic, improving the chances of the tumour ability to aggressively metastasize to other organs. Drug resistance is classified in two ways, the first being intrinsic resistance, when tumours are resistant prior to treatment, therefore the drugs do not effectively treat the tumour even with initial early diagnosis and treatment. Another form of resistance is acquired resistance which occurs despite an initial positive response to therapy (1). The mechanisms of such drug resistance mostly are as yet to be elucidated and the targets and pathways involved represent an area of intense investigation (2). For example, Martz et al. showed that activation of the mitogen-activated protein kinase (RAS-MAPK), Notch-1, phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR), PI3K/AKT and estrogen receptor (ER) signalling pathways induced resistance in a selection of different drugs. Notch-1 activation promoted acquired resistance to tamoxifen in breast cancer and to MAPK inhibitors in BRAF (V600E) melanoma cells. The use of a Notch-1 inhibitor restored sensitivity, suggesting that Notch-1 inhibition may be a therapeutic target in drug-resistant breast cancers and melanomas (3).
Myeloproliferative neoplasms (MPNs) often present an activating mutation in the gene encoding Janus kinase 2 (JAK2). Thus JAK inhibitor therapy may be of benefit for patients with MPNs containing the JAK2 (V617F) mutation. Winter and colleagues reported that when the RAS pathway is activated then resistance to JAK inhibitors is established. This is because in the sensitive cells, exposure to a JAK inhibitor induces dephosphorylation of BAD which can bind and inactivate to the prosurvival protein BCL-XL (BCL-2-like 1), triggering apoptosis. In the resistant cells, RAS pathways phosphorylate BAD in the presence of JAK inhibitors, inducing cell survival (4). Interestingly, 12 types of human papilloma viruses (HPVs) have been recently linked to cancer. HPVs have been found to inactivate p53 and retinoblastoma proteins while stimulating the PI3K/AKT, Wnt and Notch pathways. Blocking these altered signalling pathways could be critical for the eradication of HPV-associated cancers (5).
Also the epidermal growth factor receptor (EGFR) pathway seems to be involved in the resistance to chemotherapy. EGFR and PI3K/mTOR have been studied in genetically modified murine model (GEMM) and human cell lines and in a clinically relevant model of KRAS-mutant colorectal cancer (CRC) (6). The evidence suggests that inhibition of EGFR and PI3K/mTOR increases drug sensitivity, and is becoming an effective way to overcome drug resistance in cancer therapy. Furthermore, many research avenues focus on genetic and epigenetic factors that induce phenotypic changes in tumour growth and disease development. Recent evidence suggests that drug resistance is not only regulated by these factors alone but also via dysregulation of microRNAs (7).
MicroRNAs are small non-coding RNAs 19–25 nucleotides in size involved in many biological processes such as survival, apoptosis, cell cycle and gene regulation (8,9). First discovery of miRNAs was initially in 1993 when studying developmental timing in Caenorhabditis elegans (10). Mechanistically, miRNAs work by silencing gene expression and can act as both tumour suppressors and oncogenes in different types of cancer (11). miRNAs regulate gene expression through modulation of multiple target mRNAs. Perfect complementarity between the miRNA and the mRNA leads to mRNA degradation whereas imperfect complementarity gives rises to the block of translation (12).
MicroRNA biogenesis occurs in various stages involving RNA polymerase II (Pol II) which transcribes the primary transcripts (pri-miRNA). These are cleaved by the RNAse III Drosha into precursor miRNAs (pre-miRNAs) (13). The pre-miRNAs are then transported via Exportin-5 (Exp5) out of the nucleus and into the cytoplasm where they are further cleaved by Dicer, into a mature single stranded miRNA. Once the mature miRNA is excised from the pre-miRNA hairpin it is then coupled onto RNA-induced silencing complex (RISC), which induces the target mRNA to either degrade or repress the translation of mRNA targets (8).
This review primarily focuses on recent discoveries on the role of miRNAs in the resistance of different tumours to cancer drugs.
Targeting miRNAs, either decreasing or increasing their expression, seems to be an appealing stratagem for developing new and more beneficial individualized therapies, increasing drug effectiveness, and for forecasting patient response to different treatments.
Lung cancer
Lung cancer is the major cause of cancer mortality worldwide. It can be broken down into two types according to histological appearance: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). The most common is NSCLC accounting 85% of all cases (14). Irrespective of new and improved advances in the treatment of lung cancer, survival rates still remain very low, beyond 5 years, and this is due primarily to drug resistance and resistance in radiation therapy (15).
Disruption of the miRNA biogenesis pathway, Drosha, DGCR8 and Dicer leads to an increase in oncogenic mechanisms (15).
In fact reduced Dicer expression is associated with poor survival in NSCLC patients (16). Also, down-regulation of Drosha, Dicer and DGCR8 repressed miRNA maturation promoting tumorigenesis (17). A number of miRNAs have recently been identified as inducers of drug resistance in lung cancer.
We recently showed that miR-221, miR-222 and miR-30b/c are regulated by both epidermal growth factor (EGF) and MET receptors whereas miR-103 and miR-203 by just MET. These microRNAs have a role in gefitinib-induced apoptosis via inhibition of target genes such as apoptotic peptidase activating factor (APAF-1), BCL2-like11 (BIM), protein kinase C (PKC-ε) and sarcoma vital oncogene homolog (SRC). Particularly, miR-221/222 and miR-30b/c induce resistance to gefitinib by targeting APAF-1 and BIM while miR-103 and miR-203 act as tumour suppressor miRNAs in lung cancer, inducing sensitivity to TKIs and mesenchymal-epithelial transition (MET) by targeting PKC-ε and SRC, respectively (Table 1) (18). Importantly, the use of a MET inhibitor plus gefitinib significantly sensitized gefitinib resistant cells to the drug. MiR-221 and miR-222 have also been linked with resistance to TRAIL. Indeed, miR-221/222, activated by MET though the c-Jun transcription factor, induced cell migration and invasion and TRAIL resistance in lung cancer by targeting PTEN and TIMP3 and activating the AKT pathway and metallopeptidases (19). The response to TRAIL in human NSCLC has also been shown to be modulated by miR-21 (20). Thus, it is possible to theorize that the tweaking of these miRNAs with combination drug treatment could improve response to TRAIL in NSCLC.
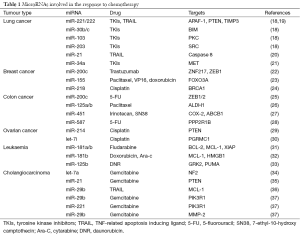
Full table
Ahmad and colleagues showed that the Hedgehog signalling plays a major role in drug resistance in lung cancer. Inhibition of this pathway using siRNAs increased the response of NSCLC cells to erlotinib, at least in part through the up-regulation of two important tumour suppressor miRNAs, miR-200b and let-7c (38). A correlation has been shown between survival to gefitinib and EGFR expression. In EGFR mutant NSCLC, miR-34a overcomes HGF-mediated gefitinib resistance by targeting MET (21) (Table 1). In another study, miR-21 over-expression was associated with acquired resistance to TKIs in NSCLC (39).
Breast cancer
Breast cancer is one of the most leading types of cancer diagnosed in women (40). Chemotherapy is considered as the most effective and important therapeutic strategy for breast cancer patients. In the last few decades, increasing knowledge of this disease gave rise to improvements in the cure; however drug resistance is still an obstacle and the underlying molecular mechanisms are mostly unknown (41).
Recently, it has been implied that miRNAs have a key role in the efficacy of chemotherapy in breast cancer (42). Zhong et al. identified 123 miRNAs that were dysregulated in vinorelbine (NVB) resistant breast cancer cell lines (MDA-MB-231/NVB). Among these 123 miRNAs, 31 of them were down-regulated and 92 miRNAs were up-regulated, suggesting that drug resistance is under the stringent control of miRNA expression (43). Furthermore, they showed that 17 specific miRNAs and their candidate targets were involved in predominant oncogenic pathways such as TGFβ, mTOR, Wnt and MAPK signalling pathways, all of them with a major role in the response to chemotherapy in breast cancer. For example, elevated TGFβ signalling and down-regulation of miR-200c has been found in trastuzumab-resistant breast cancer cells. Enforced miR-200c expression inhibited ZNF217, a transcriptional activator of TGFβ, and ZEB1 (a mediator of the TGFβ signalling). Furthermore, increased miR-200c or blockade of the TGFβ signalling synergistically promoted trastuzumab sensitivity (22) (Table 1). Loh et al. showed that AXIN2 and β-catenin, two essential mediators of the Wnt signalling were up-regulated in tamoxifen resistant cells (44). Interestingly, miR-494 and miR-141 can suppress the progression of breast cancer by repressing β-catenin expression (45,46). Kong et al. reported that miR-155 repressed the expression of Forkhead box O3a (FOXO3a) in HS578T breast cancer cells. In addition, enforced expression of miR-155 rendered BT-474 breast cells resistant to paclitaxel, VP16 and doxorubicin. Conversely, knockdown of miR-155 sensitized HS578T cells to these drugs. These interesting results suggest that miR-155 plays a crucial role in breast cancer drug resistance (23). Recently, Yu et al. reported that miR-17/20 cluster increased tamoxifen sensitivity and attenuated doxorubicin resistance in MCF-7 cells via AKT1. Over-expression of miR-17/20 was able to sensitize AKT1+/+ cells to doxorubicin (47). He et al. confirmed that miR-218 was down-regulated in cisplatin resistant breast cancer cells lines. Moreover, the expressions of miR-218 and its target breast cancer 1 (BRCA1) appears to be inversely correlated in breast cancer patients. In the same study, restoration of miR-218 sensitized MCF-7 breast cancer cells to cisplatin (24) (Table 1).
Colon cancer
CRC is the third most prevalent cause of cancer-related deaths worldwide (48). It arises as a consequence of the accumulation of genetic and epigenetic changes, which transform normal glandular epithelial cells into invasive adenocarcinoma. Only just recently, epithelial-mesenchymal transition (EMT) was reported as a potential mechanism of colon cancer development. During EMT transformed epithelial cells can acquire the abilities for tumour invasion and metastasis, resist apoptosis, and disseminate (49). Several studies showed that miRNAs are bona fide regulators of EMT process (50,51). Lee et al. reported that ectopic expression of miR-147 promoted MET in colon cancer cells, an opposite cellular program of EMT. Up-regulation of miR-147 inhibited cell proliferation and invasion, induced G1 cell cycle arrest and restored the sensitivity of the cells to gefitinib (52). Over-expression of miR-34c can reverse EMT through down-regulation of Snail in CRC cells (53). MiR-200c is another well-established miRNA involved in EMT by targeting ZEB1/2 (54). Toden et al. demonstrated that curcumin, a member of the ginger family, chemosensitizes CRC cells to 5-fluorouracil (5-FU) through the upregulation of miR-200c in 5-fluorouracil resistant (5FUR) cell lines (25).
EMT is a common feature of cancer stem cells (CSCs), which show metastatic properties and resistance to therapy. Chen et al. reported that the CSCs marker aldehyde dehydrogenase (ALDH1) is responsible for paclitaxel resistance in colon cancer. However, ectopic expression of miR-125a/b restored paclitaxel sensitivity through down-regulation of ALDH1 in HT29 cells (26). Bitarte et al. demonstrated that over-expression of miR-451 can reverse irinotecan (active metabolite) and 7-ethyl-10-hydroxy camptothecin (SN38) resistance of colon spheres in DLD1 cells via down-regulation of cyclooxygenases-2 (COX-2), which is an activator of the Wnt signalling pathway. Furthermore, ectopic expression of miR-451 has been shown to inhibit the drug transporter, ATP-binding cassette subfamily B member 1 (ABCB1) and lead to sensitization to irinotecan (27) (Table 1). In summary, the dual programme of EMT and CSCs in colon cancer may complement one another, enforcing tumour progression and metastases.
Recently, Zhang and colleagues showed that miR-587 enhanced the resistance to 5-FU via the down-regulation of the serine/threonine protein phosphatase 2A regulatory subunit 1B (PPP2R1B), an inhibitor of AKT. Silencing of PPP2R1B promoted AKT phosphorylation, up-regulated X-linked inhibitor of apoptosis protein (XIAP) and strengthened the resistance to 5-FU. Conversely, inhibition of miR-587 or enforced expression of PPP2R1B re-sensitized CRC cells to 5-FU treatment (28) (Table 1). Two interesting studies identified that insulin increased resistance to oxaliplatin, cycloheximide and 5-FU in colon cancer cells. This effect was abolished by the PI3K/AKT inhibitor LY294002. This implies that AKT plays a key role in colon cancer drug resistance (55,56). Furthermore, Chen et al. demonstrated that AKT enhanced cell growth by phosphorylating tuberous sclerosis complex 2 (TSC2) and increasing mTORC1 activity (57). Wei et al. reported that miR-302a was down-regulated in CRC cells and over-expression of miR-302a inhibited cell proliferation through the inactivation of Erk1/2 and PI3K/AKT pathways (58).
Moreover, AKT has been reported as a mediator in immune escape by activation of the immune checkpoint receptor programmed death-ligand 1 (PD-L1). Programmed death-1 (PD-1) can regulate anti-tumour immune activity through T cells antigen recognition. Activated AKT induced the expression of PD-L1. Binding of PD-L1 to PD-1 on the surface of T cells can limit T cells activity. Indeed, PD-1/PD-L1 axis induced apoptosis in antigen-specific cytotoxic T cells, therefore avoiding cancer cells elimination by T cells (57,59).
Prostate cancer
Prostate cancer is one of the leading cancers among males and the key character of prostate cancer is its androgen dependence. Therefore, androgen deprivation therapy is considered as a common approach to treat prostate cancer patients (60). However, most cases eventually develop resistance to hormone therapy becoming androgen-independent prostate cancers (AIPC) (61). Several studies correlated dysregulation of miRNAs to AIPC (62,63). Sun et al. showed that knockdown of miR-221 and miR-222 enhanced the sensitivity of cancer cells to androgen treatments (64). Another study using a high-throughput microarray approach reported that miR-21 is up-regulated in androgen receptor (AR) positive prostate cancer. AR can bind to the miR-21 promoter, revealing a direct regulation of miR-21 by AR (65). Other known tumour suppressors, miRNAs, miR-34a and miR-205 were found down-regulated in AIPC (66,67). They all target the AR; therefore, low expression of these miRNAs led to an increased expression of AR, resulting in the promotion of prostate cancer recurrence and progression. Enforced expression of these miRNAs significantly inhibited self-renewal capacity of prostate cancer cells, suggesting that their modulation may play a role in prostate cancer therapy.
Ovarian cancer
Ovarian cancer is a deadly cancer of the female reproductive system. A combination of carboplatin/cisplatin with paclitaxel is considered as a frontline therapy for ovarian cancer. However patients acquire resistance to this treatment or relapse a few years after the initial cycle of therapy (68). MiR-214 has been shown to have an important role in cisplatin resistance in certain types of ovarian cancer primarily targeting the PTEN/PI3K/AKT pathway (29). Let-7i expression is reduced in chemoresistant tumours and it has also been postulated as a causative factor for cisplatin resistance (69). One target of let-7i is progesterone receptor membrane component 1 (PGRMC1) which is part of a multi-protein complex that is over expressed in several cancers, including ovarian cancer (30) (Table 1). Kim et al. reported that miR-663 and miR-622 down-regulation increased paclitaxel sensitivity of ovarian cancer cells (70). Restoration of miR-130b decreased sensitivity to paclitaxel and cisplatin treatment by repressing P-glycoprotein (P-gp) expression (71).
Leukaemia
Currently, most of the clinical therapy for leukaemia consists of agents such as bendamustine, chlorambucil, and immunotherapeutic agents such as rituximab (72,73). However, drug resistance remains as the major obstruction. Emerging studies showed that miRNAs are involved in chemoresistance via multiple transduction pathways in leukaemia (74,75). Zhu et al. reported a novel set of 31 miRNAs that are significantly deregulated in chronic lymphocytic leukaemia (CLL). Particularly, miR-181a and miR-181b have been shown to be significantly down-regulated in CLL samples and their under-expression was associated with shorter survival and treatment-free survival. Interestingly, enforced expression of miR-181a, miR-181b and miR-34a in primary CLL cells significantly enhanced fludarabine-induced apoptosis by targeting inhibitors of apoptosis BCL-2, myeloid cell leukaemia 1 (MCL-1) and XIAP (31). Furthermore, Lu et al. showed that restoration of miR-181b increased the sensitivity of leukaemia cells to different concentrations of doxorubicin and cytarabine (Ara-C), thus enhancing apoptosis through the down-regulation of MCL-1 and High Mobility Group Box 1 (HMGB1) (32) (Table 1).
Another study demonstrated that ectopic expression of miR-125b inhibited apoptosis and induced drug resistance to daunorubicin (DNR), a chemotherapeutic agent which functions by inhibiting cell replication. MiR-125b affected the sensitivity to DNR through down-regulation of G protein-coupled receptor kinase 2 (GRK2) and p53-upregulated modulator of apoptosis (PUMA), which contribute to cell apoptosis by enhancing caspase-3 cleavage (33) (Table 1).
Cholangiocarcinoma
Cholangiocarcinoma is resistant to drug therapy (76). Meng and colleagues showed that IL-6-activated survival pathways contributed to tumour growth and resistance to therapy in this cancer. IL-6 induced upregulation of let-7, which caused an increase in Stat-3 phosphorylation by targeting the neurofibromatosis 2 (NF2) gene (34). In a different study, Meng et al. showed that miR-21 and miR-200b were involved in resistance to gemcitabine; inhibition of these miRNAs reversed this resistant phenotype. MiR-21 modulated the response to gemcitabine through PTEN down-regulation and consequent activation of the PI3K pathway (35). One of the mechanisms of TRAIL resistance to cancer therapy is MCL-1 up-regulation (77). Mott and colleagues showed that enforced expression of miR-29b reduced MCL-1 protein levels, thereby increasing sensitivity of cholangiocarcinoma cells to TRAIL-induced apoptosis (36). Furthermore miR-29b, miR-205 and miR-221 enhanced chemo-sensitivity to gemcitabine in HuH28 human cholangiocarcinoma cells by targeting phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1) (miR-29b and miR-221) or matrix metallopeptidase 2 (MMP-2) (miR-29b) (37) (Table 1).
Concluding remarks
With drug resistance remaining a significant setback in the clinical setting, leading to relapse and metastatic spread in many cancer types, new therapeutic strategies are urgently needed. miRNAs have opened up a new avenue in the understanding of the molecular mechanisms involved in cancer, raising the hope of developing new and more effective therapeutic strategies. miRNAs modulate multiple signalling pathways and regulatory networks, so that even subtle changes in miRNAs expression can cause significant changes in disease progression and cancer outcome. Many groups are investigating the use of microRNAs as potential therapeutic agents. The research is now being more focused on in vivo and translational studies.
There is evidence that suggests that miRNAs might be potential therapeutic tools especially in combination with anti-cancer chemotherapeutics. This could be in the form of antagomiRs that silence miRNA expression, or mimics that reinforce the function and expression of miRNAs. miRNA mimics or anti-miRNAs, by influencing the expression of endogenous microRNAs in tumour cells have the potential to alter chemotherapy effectiveness. Two clinical studies have demonstrated a future potential impact of miRNAs as therapeutics. These include a phase 2a clinical trial using Miravirsen (a nucleic acid—modified DNA phosphorothioate antisense oligonucleotide that encapsulates mature miR-122 in a heteroduplex thereby inhibiting its function) in 26 patients infected with chronic hepatitis C virus (HCV) genotype 1. Thus far no adverse effects have been reported during the trial (78). Another clinical trial currently in phase 1 involves MRX34 (a mimic of the tumour suppressor miR-34) in patients with liver cancer or metastatic cancer with liver complications. In this trial the safety and effects of MRX34 are being evaluated in healthy volunteers and patients with advanced or metastatic liver cancer (hepatocellular carcinoma) (79). Future prospects for these new therapies are positive because the preliminary results in both trials look promising.
However there are still many difficulties to overcome before we should be able to use miRNAs in the clinical setting. The main obstacle is the delivery system. Chemical modifications and the use of viral vectors or nanoparticle might help us in overcome this hurdle. Despite these delivery pitfalls, it can be postulated that there is a major role for miRNAs in the future of cancer therapy. miRNA treatments alongside traditional chemotherapeutic modalities and drug targets altogether provide a new strategy to treat cancer, although further research is required into this promising paradigm before progressing into clinic.
Acknowledgements
This work was supported by Cancer Research UK.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rebucci M, Michiels C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem Pharmacol 2013;85:1219-26. [PubMed]
- Szakács G, Paterson JK, Ludwig JA, et al. Targeting multidrug resistance in cancer. Nat Rev Drug Discov 2006;5:219-34. [PubMed]
- Martz CA, Ottina KA, Singleton KR, et al. Systematic identification of signaling pathways with potential to confer anticancer drug resistance. Sci Signal 2014;7:ra121. [PubMed]
- Winter PS, Sarosiek KA, Lin KH, et al. RAS signaling promotes resistance to JAK inhibitors by suppressing BAD-mediated apoptosis. Sci Signal 2014;7:ra122. [PubMed]
- Chen J. Signaling pathways in HPV-associated cancers and therapeutic implications. Rev Med Virol 2015;25 Suppl 1:24-53. [PubMed]
- Belmont PJ, Jiang P, McKee TD, et al. Resistance to dual blockade of the kinases PI3K and mTOR in KRAS-mutant colorectal cancer models results in combined sensitivity to inhibition of the receptor tyrosine kinase EGFR. Sci Signal 2014;7:ra107. [PubMed]
- Fojo T. Multiple paths to a drug resistance phenotype: mutations, translocations, deletions and amplification of coding genes or promoter regions, epigenetic changes and microRNAs. Drug Resist Updat 2007;10:59-67. [PubMed]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281-97. [PubMed]
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006;6:857-66. [PubMed]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993;75:843-54. [PubMed]
- Ventura A, Jacks T. MicroRNAs and cancer: short RNAs go a long way. Cell 2009;136:586-91. [PubMed]
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 2004;5:522-31. [PubMed]
- Lee Y, Ahn C, Han J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003;425:415-9. [PubMed]
- Oser MG, Niederst MJ, Sequist LV, et al. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol 2015;16:e165-72. [PubMed]
- MacDonagh L, Gray SG, Finn SP, et al. The emerging role of microRNAs in resistance to lung cancer treatments. Cancer Treat Rev 2015;41:160-9. [PubMed]
- Karube Y, Tanaka H, Osada H, et al. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci 2005;96:111-5. [PubMed]
- Kumar MS, Lu J, Mercer KL, et al. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet 2007;39:673-7. [PubMed]
- Garofalo M, Romano G, Di Leva G, et al. EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat Med 2011;18:74-82. [PubMed]
- Garofalo M, Di Leva G, Romano G, et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell 2009;16:498-509. [PubMed]
- Jeon YJ, Middleton J, Kim T, et al. A set of NF-κB-regulated microRNAs induces acquired TRAIL resistance in lung cancer. Proc Natl Acad Sci U S A 2015;112:E3355-64. [PubMed]
- Zhou JY, Chen X, Zhao J, et al. MicroRNA-34a overcomes HGF-mediated gefitinib resistance in EGFR mutant lung cancer cells partly by targeting MET. Cancer Lett 2014;351:265-71. [PubMed]
- Bai WD, Ye XM, Zhang MY, et al. MiR-200c suppresses TGF-β signaling and counteracts trastuzumab resistance and metastasis by targeting ZNF217 and ZEB1 in breast cancer. Int J Cancer 2014;135:1356-68. [PubMed]
- Kong W, He L, Coppola M, et al. MicroRNA-155 regulates cell survival, growth, and chemosensitivity by targeting FOXO3a in breast cancer. J Biol Chem 2010;285:17869-79. [PubMed]
- He X, Xiao X, Dong L, et al. MiR-218 regulates cisplatin chemosensitivity in breast cancer by targeting BRCA1. Tumour Biol 2015;36:2065-75. [PubMed]
- Toden S, Okugawa Y, Jascur T, et al. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 2015;36:355-67. [PubMed]
- Chen J, Chen Y, Chen Z. MiR-125a/b regulates the activation of cancer stem cells in paclitaxel-resistant colon cancer. Cancer Invest 2013;31:17-23. [PubMed]
- Bitarte N, Bandres E, Boni V, et al. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem Cells 2011;29:1661-71. [PubMed]
- Zhang Y, Talmon G, Wang J. MicroRNA-587 antagonizes 5-FU-induced apoptosis and confers drug resistance by regulating PPP2R1B expression in colorectal cancer. Cell Death Dis 2015;6:e1845.
- Yang H, Kong W, He L, et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res 2008;68:425-33. [PubMed]
- Wendler A, Keller D, Albrecht C, et al. Involvement of let-7/miR-98 microRNAs in the regulation of progesterone receptor membrane component 1 expression in ovarian cancer cells. Oncol Rep 2011;25:273-9. [PubMed]
- Zhu DX, Zhu W, Fang C, et al. miR-181a/b significantly enhances drug sensitivity in chronic lymphocytic leukemia cells via targeting multiple anti-apoptosis genes. Carcinogenesis 2012;33:1294-301. [PubMed]
- Lu F, Zhang J, Ji M, et al. miR-181b increases drug sensitivity in acute myeloid leukemia via targeting HMGB1 and Mcl-1. Int J Oncol 2014;45:383-92. [PubMed]
- Zhou L, Bai H, Wang C, et al. microRNA-125b promotes leukemia cell resistance to daunorubicin by inhibiting apoptosis. Mol Med Rep 2014;9:1909-16. [PubMed]
- Meng F, Henson R, Wehbe-Janek H, et al. The MicroRNA let-7a modulates interleukin-6-dependent STAT-3 survival signaling in malignant human cholangiocytes. J Biol Chem 2007;282:8256-64. [PubMed]
- Meng F, Henson R, Lang M, et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology 2006;130:2113-29. [PubMed]
- Mott JL, Kobayashi S, Bronk SF, et al. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 2007;26:6133-40. [PubMed]
- Okamoto K, Miyoshi K, Murawaki Y. miR-29b, miR-205 and miR-221 enhance chemosensitivity to gemcitabine in HuH28 human cholangiocarcinoma cells. PLoS One 2013;8:e77623. [PubMed]
- Ahmad A, Maitah MY, Ginnebaugh KR, et al. Inhibition of Hedgehog signaling sensitizes NSCLC cells to standard therapies through modulation of EMT-regulating miRNAs. J Hematol Oncol 2013;6:77. [PubMed]
- Seike M, Goto A, Okano T, et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci U S A 2009;106:12085-90. [PubMed]
- DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin 2014;64:52-62. [PubMed]
- Nwabo Kamdje AH, Seke Etet PF, Vecchio L, et al. Signaling pathways in breast cancer: therapeutic targeting of the microenvironment. Cell Signal 2014;26:2843-56. [PubMed]
- Tian W, Chen J, He H, et al. MicroRNAs and drug resistance of breast cancer: basic evidence and clinical applications. Clin Transl Oncol 2013;15:335-42. [PubMed]
- Zhong S, Ma T, Zhang X, et al. MicroRNA expression profiling and bioinformatics analysis of dysregulated microRNAs in vinorelbine-resistant breast cancer cells. Gene 2015;556:113-8. [PubMed]
- Loh YN, Hedditch EL, Baker LA, et al. The Wnt signalling pathway is upregulated in an in vitro model of acquired tamoxifen resistant breast cancer. BMC Cancer 2013;13:174. [PubMed]
- Song L, Liu D, Wang B, et al. miR-494 suppresses the progression of breast cancer in vitro by targeting CXCR4 through the Wnt/β-catenin signaling pathway. Oncol Rep 2015;34:525-31. [PubMed]
- Abedi N, Mohammadi-Yeganeh S, Koochaki A, et al. miR-141 as potential suppressor of β-catenin in breast cancer. Tumour Biol 2015. [Epub ahead of print]. [PubMed]
- Yu Z, Xu Z, Disante G, et al. miR-17/20 sensitization of breast cancer cells to chemotherapy-induced apoptosis requires Akt1. Oncotarget 2014;5:1083-90. [PubMed]
- Ricci-Vitiani L, Fabrizi E, Palio E, et al. Colon cancer stem cells. J Mol Med (Berl) 2009;87:1097-104. [PubMed]
- De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013;13:97-110. [PubMed]
- Hur K, Toiyama Y, Takahashi M, et al. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut 2013;62:1315-26. [PubMed]
- Tian Y, Pan Q, Shang Y, et al. MicroRNA-200 (miR-200) cluster regulation by achaete scute-like 2 (Ascl2): impact on the epithelial-mesenchymal transition in colon cancer cells. J Biol Chem 2014;289:36101-15. [PubMed]
- Lee CG, McCarthy S, Gruidl M, et al. MicroRNA-147 induces a mesenchymal-to-epithelial transition (MET) and reverses EGFR inhibitor resistance. PLoS One 2014;9:e84597. [PubMed]
- Siemens H, Jackstadt R, Hünten S, et al. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle 2011;10:4256-71. [PubMed]
- Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008;10:593-601. [PubMed]
- Chen J, Huang XF, Qiao L, et al. Insulin caused drug resistance to oxaliplatin in colon cancer cell line HT29. J Gastrointest Oncol 2011;2:27-33. [PubMed]
- Chen J, Katsifis A, Hu C, et al. Insulin decreases therapeutic efficacy in colon cancer cell line HT29 via the activation of the PI3K/Akt pathway. Curr Drug Discov Technol 2011;8:119-25. [PubMed]
- Chen J, Zhang XD, Proud C. Dissecting the signaling pathways that mediate cancer in PTEN and LKB1 double-knockout mice. Sci Signal 2015;8:pe1. [PubMed]
- Wei ZJ, Tao ML, Zhang W, et al. Up-regulation of microRNA-302a inhibited the proliferation and invasion of colorectal cancer cells by regulation of the MAPK and PI3K/Akt signaling pathways. Int J Clin Exp Pathol 2015;8:4481-91. [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [PubMed]
- Lauer RC, Friend SC, Rietz C, et al. Drug design strategies for the treatment of prostate cancer. Expert Opin Drug Discov 2015;10:81-90. [PubMed]
- Li F, Mahato RI. MicroRNAs and drug resistance in prostate cancers. Mol Pharm 2014;11:2539-52. [PubMed]
- Ma S, Chan YP, Kwan PS, et al. MicroRNA-616 induces androgen-independent growth of prostate cancer cells by suppressing expression of tissue factor pathway inhibitor TFPI-2. Cancer Res 2011;71:583-92. [PubMed]
- Shi GH, Ye DW, Yao XD, et al. Involvement of microRNA-21 in mediating chemo-resistance to docetaxel in androgen-independent prostate cancer PC3 cells. Acta Pharmacol Sin 2010;31:867-73. [PubMed]
- Sun T, Wang Q, Balk S, et al. The role of microRNA-221 and microRNA-222 in androgen-independent prostate cancer cell lines. Cancer Res 2009;69:3356-63. [PubMed]
- Ribas J, Ni X, Haffner M, et al. miR-21: an androgen receptor-regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res 2009;69:7165-9. [PubMed]
- Kashat M, Azzouz L, Sarkar SH, et al. Inactivation of AR and Notch-1 signaling by miR-34a attenuates prostate cancer aggressiveness. Am J Transl Res 2012;4:432-42. [PubMed]
- Hagman Z, Haflidadóttir BS, Ceder JA, et al. miR-205 negatively regulates the androgen receptor and is associated with adverse outcome of prostate cancer patients. Br J Cancer 2013;108:1668-76. [PubMed]
- Davidson B, Tropé CG. Ovarian cancer: diagnostic, biological and prognostic aspects. Womens Health (Lond Engl) 2014;10:519-33. [PubMed]
- Yang N, Kaur S, Volinia S, et al. MicroRNA microarray identifies Let-7i as a novel biomarker and therapeutic target in human epithelial ovarian cancer. Cancer Res 2008;68:10307-14. [PubMed]
- Kim YW, Kim EY, Jeon D, et al. Differential microRNA expression signatures and cell type-specific association with Taxol resistance in ovarian cancer cells. Drug Des Devel Ther 2014;8:293-314. [PubMed]
- Zong C, Wang J, Shi TM. MicroRNA 130b enhances drug resistance in human ovarian cancer cells. Tumour Biol 2014;35:12151-6. [PubMed]
- Chang JE, Kahl BS. Bendamustine for treatment of chronic lymphocytic leukemia. Expert Opin Pharmacother 2012;13:1495-505. [PubMed]
- Foà R, Del Giudice I, Cuneo A, et al. Chlorambucil plus rituximab with or without maintenance rituximab as first-line treatment for elderly chronic lymphocytic leukemia patients. Am J Hematol 2014;89:480-6. [PubMed]
- Zenz T, Mohr J, Eldering E, et al. miR-34a as part of the resistance network in chronic lymphocytic leukemia. Blood 2009;113:3801-8. [PubMed]
- Asslaber D, Piñón JD, Seyfried I, et al. microRNA-34a expression correlates with MDM2 SNP309 polymorphism and treatment-free survival in chronic lymphocytic leukemia. Blood 2010;115:4191-7. [PubMed]
- Shen DY, Zhang W, Zeng X, et al. Inhibition of Wnt/β-catenin signaling downregulates P-glycoprotein and reverses multi-drug resistance of cholangiocarcinoma. Cancer Sci 2013;104:1303-8. [PubMed]
- Taniai M, Grambihler A, Higuchi H, et al. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res 2004;64:3517-24. [PubMed]
- Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med 2013;368:1685-94. [PubMed]
- Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov 2013;12:847-65. [PubMed]


