Outcomes of reirradiation in the treatment of patients with multiple brain metastases of solid tumors: a retrospective analysis
Introduction
Around 20–40% of cancer patients are estimated to develop brain metastases during the course of their disease (1). Due to the improved systemic therapy and longer patient survival, brain metastasis is an increasingly common problem. Prognosis is generally poor for these patients and median survival is 4–5 months after diagnosis (2). While the treatment options are surgery, stereotactic radiosurgery and chemotherapy, the standard therapy for patients with multiple brain metastases is still whole brain radiation therapy (WBRT) (3,4). WBRT provides effective palliation for neurologic symptoms, improves patients’ quality of life and can prolong survival (5) but, the median time to clinical progression is between 6 and 13 weeks after WBRT (6). In the recurrent situation, patients with multiple metastatic lesions, treatment options are limited, including re-irradiation WBRT (re-WBRT), best supportive care, surgery and chemotherapy. The treatment schema must be decided individually based on the patient’s performance status, number of metastases, primary diagnosis, previous treatments and extracranial disease (7).
Re-WBRT is an important treatment option for patients with stable extracranial disease and sufficient performance status. However, radiation toxicity is very important in re-WBRT and various factors including treated tissue volume, total cumulative radiation dose, fractionation scheme, interval of time between first and second course may influence it (8,9). A systematic search of the National Library of Medicine (PubMed) stated that comparative analyses are missing and patient numbers in the existing studies (8,10-17) are not sufficient to lead to general recommendations.
In this retrospective study, we present outcomes of re-WBRT in the treatment of patients with multiple brain metastases of solid tumors.
Methods
The design of the present study was approved by the Ethical Committee and Institutional Review Board of Necmettin Erbakan University Faculty of Medicine, where the study was conducted.
We identified 34 adult patients in our center database that had received re-WBRT due to multiple brain metastases in our department between June 2010 and June 2014. Patients with metastases of the skull or primary brain tumors were excluded. We also excluded that who had received partial brain radiotherapy (RT) or not WBRT, e.g., stereotactic radiosurgery in the second course RT.
Using the patients charts, the fallowing data was obtained: sex, age, number of brain metastases, surgical resection, primary tumor site, symptoms including ataxia, headache, neurocognitive and visual changes, recursive partitioning analysis (RPA), RT dose of the first and second WBRT, biological effective dose (BED), Karnofsky performance status (KPS) before first and second course RT, steroid using, time between radiation courses, symptomatic response to RT and survival time.
All patients were assigned a RPA class based on KPS, local control, extracranial metastases status and age. Patients ≤65, with a KPS ≥70, local control and no extracranial metastases were assigned RPA class I. Patients with a KPS <70 were assigned RPA class III, all others were assigned to group II.
BED was calculated using the linear quadratic model: {BED = nd [1 + d/(a/b)]} in grays, where d = fraction dose (in grays), n = number of fractions, nd = D = total physical dose (in grays), and a/b is the ratio of 2 Gy (18). The cumulative BED (BEDcumulative) was calculated by the addition of the BED of the first and second courses of WBRT.
Survival time was defined as the time from the diagnosis of brain metastasis (OS1), from the start of the second course of WBRT (OS2) and as the time from the diagnosis of primary tumor (OS3) to date of the death. Date of death was obtained from medical and official records or was learned by phone.
Statistical analyses were performed with SPSS version 18.0.1 (SPSS Inc., Chicago, IL, USA) using Cox regression analyses (stepwise backwards, Pin 0.05, Pout 0.1), log-rank test and Kaplan-Meier’s method. Statistical significance was defined as P<0.05.
Results
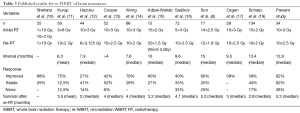
Thirty four patients received a second course of WBRT in our institution between June 2010 and June 2014. The median age at the start of re-WBRT was 60 years (range, 32–76 years). All of these patients were noted to have multiple brain metastases and they were not suitable to treatment with stereotactic radiosurgery. Six patients underwent resection of intracranial disease at some point before the first course of RT and two patients before the re-WBRT. The main primary tumor site was lung (22 of 34 patients, 64.7%) followed by breast (7 of 34 patients, 20.6%) and others (1 malignant melanoma, 1 multiple myeloma, 1 renal cell carcinoma and 1 larynx carcinoma, 1 maxillary sinus tumor). Patient characteristics presented in Table 1.
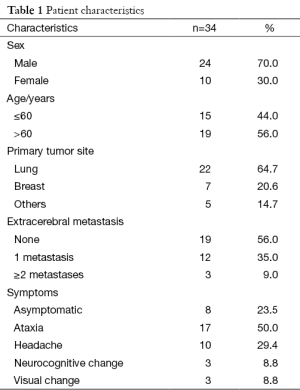
Full table
Thirteen patients (38%) had severe symptoms, 13 patients (38%) had mild symptoms and 8 patients (24%) were asymptomatic.
Based on KPS, age, local control and extracranial metastasis at the time of re-WBRT, the majority of patients (19 patients, 56%) were classified as RPA class II, 13 patients (38%) were classified as RPA class I and 2 patients (6%) were classified as RPA class III.
The median dose for the first WBRT was 30 Gy (range, 25–30 Gy).The median dose for the second WBRT was 25 Gy (range, 20–30 Gy).The median BED dose at the first course of RT was 75 Gy (range, 62.5–75 Gy) and 62.5 Gy (range, 48–75 Gy) at the initiation of re-WBRT. The median BEDcumulative was 137.5 Gy (range, 110.5–150 Gy).
The median KPS was 90 (range, 60–100) at the time of first course RT and 80 (range, 50–100) at the initiation of re-WBRT.
Twenty patients (59%) were given steroids either before or during the re-WBRT. The median daily dose of the steroids was 8 mg (range, 4–24 mg daily).
The median time interval between the first and the second WBRT was 12.8 months (range, 5.8–45.7 months).
At the end of the therapy 38% of patients showed progression of symptoms, 38% of patients were stable or partial regression and 24% showed clinical regression.
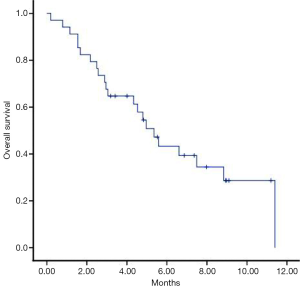
The median overall survival from the initial diagnosis of brain metastasis (OS1) was 20.8 months (95% CI, 16.63–24.10), after the re-WBRT (OS2) it was 5.3 months (95% CI, 4.08–6.62) (Figure 1) from the diagnosis of primary tumor (OS3) to date of the death was 27.1 months (95% CI, 17.75-37.04).
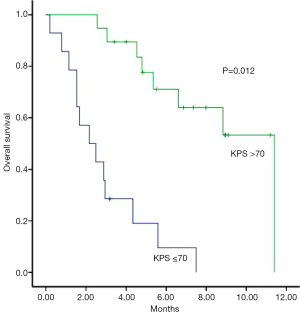
In multivariate analysis, patients with a KPS of >70 had a median survival of 11.4 months, compared to 2.2 months for those with a KPS of ≤70 (P=0.012) (Figure 2) and patients who have severe symptoms at the time of re-WBRT with median survival 2.2 months while those with mild symptoms had a median of 4.8 months survival (P=0.08) and all of the asymptomatic patients are still alive (Figure 3). Age, gender, primary tumor site, RPA class, interval between two course of WBRT, BEDcumulative, extra cerebral metastasis and response to re-WBRT did not have any predictive value (Table 2).

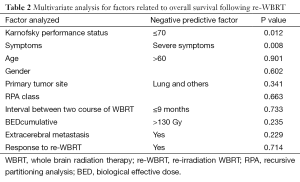
Full table
Discussion
In this present study, our survival data indicates that re-WBRT may be useful in patients with a good performance status and mild or no symptoms and our results similar to the findings in the current literature (17,19).
There have been a limited number of articles in the literature describing re-WBRT with acceptable toxicities, minimal side effects and a treatment that provides symptomatic relief. We have summarized our results to show an overview of previously published studies related with re-WBRT in Table 3.
Full table
Several retrospective studies have examined the effects of re-WBRT in patients with multiple brain metastases, the patient numbers in the existing studies are low (8,10-17). Scharp et al. were analyzed the largest patients numbers with 134 patients (19), Wong et al. with 86 patients (14) and Sadikov et al. with 72 patients (16). We present this study with 34 patients.
Our median of 30 Gy in 15 fractions for the initial RT is in accordance with most of the other studies (12-14) in terms of doses and number of fractions used. Only Shehata et al. used a one-time irradiation of 10 Gy (10) for the re-WBRT, whereas all other cases used 8–12 fractions (8,11,13,14,16,17,19). We have not observed a positive correlation between dose increment and response to treatment in our patients with BEDcumulative of almost 130 Gy, and we applied 25 Gy in 10 fractions in the majority of cases for the re-WBRT.
Associations between re-WBRT and historically favorable prognostic factors were also examined in previous studies including performance status (KPS >70), control of primary disease, absence of extracranial metastases, and younger age (<65 years) as defined RPA classes (20). Cooper et al. attributed their favorable outcomes to their patient population, who were with controlled primary disease and limited extracranial metastases and more likely to be younger (mean age, 57.3 years) (13).
In this study, our patient population was quite favorable, with a median initial KPS of 80 and mean age of 60 years. We found that patients with KPS >70 had an improved survival compared to those with KPS ≤70 (P=0.012). Sadikov et al. showed no difference in overall survival between patients with extracranial disease compared to those without extracranial metastases or active primary disease (16). In our study we did not find any correlation with the extracranial disease status like Sadikov et al.
There is limited data to support an ideal time interval between radiation courses.
The median time interval between first and second course RT in our study was 12.8 months and this interval was longer than the most previous studies except for Son et al. with 15 months (8), similar to Sadikov et al. (16), the time between radiation courses did not affect overall survival in our study.
Response rates are different between the analyses. Son et al. (8) found improved symptoms in 80% of cases, but Hazuka and Kinzie (12) showed rate of improved symptoms in 27% of cases. These variances are probably based on doctor’s notes patient charts and also subjectivity of symptoms. In our study, 38% of patients showed progression of symptoms, 38% of patients were stable or partial regression and 24% showed clinical regression.
In our study, many patients were receiving steroid therapy at any time of radiation therapy. Steroid use was not well documented in previous studies. Because of the differences in reasoning for steroid initiation (symptom palliation or prophylaxis) and the variations in daily dose throughout a patient’s treatment course, it is difficult to characterize the extent of steroid role in symptom palliation.
The median survival time after the second course WBRT ranged from 2 to 5.2 months in previous studies. In our study, the median survival was 5.3 months, which were similar to the findings in the current literature.
There are some limitations in our study. First limitation of our study was that toxicity rates and acute or late side effects have not been very well documented after re-WBRT because of the difficulty in interpreting patient charts retrospectively and a relatively small number of patients alive at a longer follow-up. Another limitation in assessing side effects is the difficulty in determining whether these symptoms were caused by the progressive metastases or WBRT. However, based on our follow-up notes and data, none of our patients suffered from severe neurotoxicity or died due to re-WBRT. Further study on toxicity analyses following reirradiation can help to determine the role of this treatment.
Overall survival after re-WBRT was 5.3 months in our study and we did not see severe neurotoxicity. As a conclusion, our survival data indicates that patients who have good performance status and who do not have severe symptoms might benefit from re-WBRT and re-WBRT seems to be associated with minimal toxicity in patients treated with lower total and fractional palliation doses.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Soffietti R, Rudà R, Trevisan E. Brain metastases: current management and new developments. Curr Opin Oncol 2008;20:676-84. [PubMed]
- Lagerwaard FJ, Levendag PC, Nowak PJ, et al. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys 1999;43:795-803. [PubMed]
- Gaspar LE, Mehta MP, Patchell RA, et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2010;96:17-32. [PubMed]
- Hauswald H, Dittmar JO, Habermehl D, et al. Efficacy and toxicity of whole brain radiotherapy in patients with multiple cerebral metastases from malignant melanoma. Radiat Oncol 2012;7:130. [PubMed]
- Scoccianti S, Ricardi U. Treatment of brain metastases: review of phase III randomized controlled trials. Radiother Oncol 2012;102:168-79. [PubMed]
- Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1980;6:1-9. [PubMed]
- Ammirati M, Cobbs CS, Linskey ME, et al. The role of retreatment in the management of recurrent/progressive brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2010;96:85-96. [PubMed]
- Son CH, Jimenez R, Niemierko A, et al. Outcomes after whole brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys 2012;82:e167-72. [PubMed]
- Mayer R, Sminia P. Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys 2008;70:1350-60. [PubMed]
- Shehata WM, Hendrickson FR, Hindo WA. Rapid fractionation technique and retreatment of cerebral metastases by irradiation. Cancer 1974;34:257-61. [PubMed]
- Kurup P, Reddy S, Hendrickson FR. Results of re-irradiation for cerebral metastases. Cancer 1980;46:2587-9. [PubMed]
- Hazuka MB, Kinzie JJ. Brain metastases: results and effects of re-irradiation. Int J Radiat Oncol Biol Phys 1988;15:433-7. [PubMed]
- Cooper JS, Steinfeld AD, Lerch IA. Cerebral metastases: value of reirradiation in selected patients. Radiology 1990;174:883-5. [PubMed]
- Wong WW, Schild SE, Sawyer TE, et al. Analysis of outcome in patients reirradiated for brain metastases. Int J Radiat Oncol Biol Phys 1996;34:585-90. [PubMed]
- Abdel-Wahab MM, Wolfson AH, Raub W, et al. The role of hyperfractionated reirradiation in metastatic brain disease: a single institutional trial. Am J Clin Oncol 1997;20:158-60. [PubMed]
- Sadikov E, Bezjak A, Yi QL, et al. Value of whole brain re-irradiation for brain metastases-single centre experience. Clin Oncol (R Coll Radiol) 2007;19:532-8. [PubMed]
- Ozgen Z, Atasoy BM, Kefeli AU, et al. The benefit of whole brain reirradiation in patients with multiple brain metastases. Radiat Oncol 2013;8:186. [PubMed]
- Fowler JF. 21 years of biologically effective dose. Br J Radiol 2010;83:554-68. [PubMed]
- Scharp M, Hauswald H, Bischof M, et al. Re-irradiation in the treatment of patients with cerebral metastases of solidtumors: retrospective analysis. Radiat Oncol 2014;9:4. [PubMed]
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [PubMed]


