The results with the addition of metronomic cyclophosphamide to palliative radiotherapy for the treatment of non-small cell lung carcinoma
Introduction
Pulmonary adenocarcinoma and squamous cell carcinoma are the two major variants of lung cancer which are covered under the blanket term ‘non-small cell lung carcinoma’ (NSCLC). The currently accepted standards of care for NSCLC include radical surgery, radical chemotherapy, concurrent chemoradiotherapy, stereotactic radiotherapy (RT) and molecular targeted therapies. However, in reality a large proportion of NSCLC patients are ineligible for radical therapies owing to the oft present issues of debilitating co-morbidities, advanced age and poor performance status. A significant proportion of NSCLC patients who are inoperable due to any reason ultimately either receive palliative treatments, or supportive care alone (1-4).
The palliative treatment approaches traditionally included palliative RT, palliative intravenous chemotherapy, or both. More recently, the availability of oral tyrosine kinase inhibitors (such as gefitinib and crizotinib) has revolutionized treatment of NSCLC. However though very effective, these targeted therapies are applicable only in certain mutational variants of adenocarcinoma (5). Also being expensive, targeted therapies are not often afforded by a large proportion of patients (6). It must be mentioned here that there are no effective targeted therapies for squamous cell carcinoma, yet (7).
Palliative short course RT has various advantages over palliative chemotherapy, in that there are no significant systemic toxicities of localized RT, that RT can be truncated in case of non-tolerability, and that RT provides quick symptomatic relief from symptoms such as superior venocaval obstruction (8,9). Our institutional protocol for palliative care in NSCLC is to use palliative RT first when primary treatment with palliative intravenous chemotherapy or targeted therapies is not feasible.
In the past decade, there has been a vigorous interest in the use of metronomic chemotherapy, especially in the difficult clinical situations involving patients who are unlikely to tolerate more intense forms of conventional treatments, or in those who have already exhausted conventional treatments (10,11). Metronomic chemotherapy is unique in that repeated administration of low doses of chemotherapy is performed under a chronic tolerable schedule (12).
After April 2013, owing to the then strong zeitgeist favoring the use of metronomic chemotherapy, and its attractive features of considerable efficacy at low toxicity, we made it an institutional policy to use oral low dose metronomic cyclophosphamide as part of standard therapy for all NSCLC patients who could not tolerate systemic intravenous conventional chemotherapy or targeted therapy. Owing to the very low toxicity, it could be easily used alongside palliative RT.
By mid-2015, we realized an unique retrospective opportunity of evaluating the effect of adding metronomic oral cyclophosphamide to palliative RT for NSCLC, by comparing patients treated after April 1 2013 vs. those treated prior to April 2013.
Material and methods
A total of 184 patient records treated between the time span of January 2011 to December 2014 fulfilled the eligibility criteria (the inclusion and exclusion criteria presented in Table 1). Of these, 16 were excluded since the patients had received additional forms of palliative chemotherapy, 9 were excluded for having received oral targeted therapies during follow up, 6 were excluded due to ambiguous histologies such as adenosquamous variety, and 14 were excluded due to incomplete follow-up records.

Full table
Throughout this study, group A refers to the set of NSCLC patients who received palliative RT alone, during the time span of January 1 2011 to March 31 2013. Group B refers to the set of patients who received oral metronomic cyclophosphamide (50 mg per day, beginning from the day of initiation of RT until progression) in addition to palliative RT, the time span being from April 1 2013 to 31 December 2014.
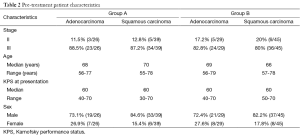
The initial patient characteristics at presentation were comparable in the two groups (Table 2). The dose of palliative RT ranged from 20-30 Grays delivered over 5-10 fractions, and was similar for both the groups.
Full table
Response was assessed as per the RECIST criteria, via contrast enhanced computed tomography scans performed at around 1 month after completion of palliative RT (13). Patients with complete response or partial response would be recorded as ‘responders’, whereas patients with stable disease or progression would be recorded as non-responders. Response rates of the two groups were compared using the Fisher-exact-test.
Progression free survival (PFS) was the main evaluation in this study. Progression would be declared when existing lesions either increased in size, or if there were development of newer disease foci which could be nodal or metastatic, or if there were development of malignant effusions. The PFS for both groups were plotted as Kaplan-Meier survival curves, and the curves were compared using the log-rank test.
The cut-off for statistical significance was placed at P≤0.05. The data mined from hospital patient records were consolidated into an Open Document Format spreadsheet and subsequent statistical evaluations were performed using Gnumeric 1.1. All patient data was masked, and at no time has been any patient’s details been compromised. This study conforms to the declaration of Helsinki from the ethical perspective.
Results
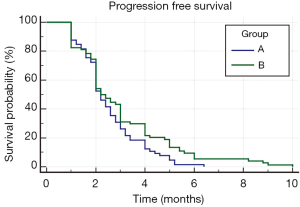
The mean PFS in the group of patients who received metronomic oral cyclophosphamide in addition to palliative RT (group B) was 3.1 months (SE 0.235; 95% CI, 2.59-3.52). This was significantly higher in comparison to that for patients treated with palliative RT alone (group A) who had a mean PFS of 2.55 months (SE 0.152; 95% CI, 2.24-2.84), the difference being statistically significant (P=0.0501) (Figure 1).
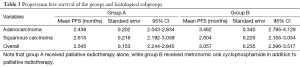
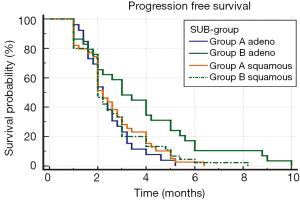
When PFS was analyzed with regards to histology (Table 3), the patients with adenocarcinoma histology from group-B had better PFS (3.5 months, SE 0.34; 95% CI, 2.79-4.12) in comparison to adenocarcinoma histology patients from group A (2.4 months, SE 0.202; 95% CI, 2.04-2.83). The difference was statistically very significant (P=0.0053). On the other hand, for patients with squamous cell histology, the PFS for patients from group B (2.6 months, SE 0.229; 95% CI, 2.155-3.054) was no different than for those from group A (2.6 months, SE 0.216; 95% CI, 2.192-3.039). Thus, histological subgroup analysis revealed that the PFS benefit from the adding of metronomic oral cyclophosphamide to palliative RT was limited only to the adenocarcinoma sub-group (Figure 2).
Full table
The response rates tended to be improved in group B in comparison to group A (41.9% vs. 33.9%), though statistical significance could not be reached (P=0.3831). This trend towards enhancement in response rate was also dependent upon histology. Metronomic oral cyclophosphamide added to palliative RT for patients of adenocarcinoma histology tended to enhance response rates in comparison to treatment with palliative RT alone (55.2% vs. 34.6%, P=0.1767). On the other hand, the addition of oral metronomic CPA to palliative RT had no effect for patients of the squamous cell carcinoma sub-groups (Table 4).
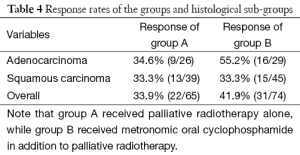
Full table
There was no incidence of hematological toxicity attributed to the use of oral cyclophosphamide at a dose of 50 mg once a day for prolonged periods. Nor were radiation induced toxicities exacerbated with the addition of oral low dose cyclophosphamide.
Thus the results can be summarized that there was a statistically significant improvement in PFS, and a trend towards improvement in response rates with the addition of oral metronomic cyclophosphamide to palliative RT, with the benefits restricted to patients with adenocarcinoma histology.
Discussion
Metronomic chemotherapy is the chronic administration of chemotherapy at low doses which are minimally toxic, in a schedule of administration without prolonged drug free breaks (12). Commonly used agents include low dose versions of conventional agents such as cyclophosphamide, methotrexate, etoposide and others (12). In contrast to the routinely used conventional chemotherapy delivered at maximally tolerated doses (MTD), the use of metronomic chemotherapy is performed with low doses and hence is associated with very low toxicity. This makes metronomic chemotherapy a very attractive option in patients who cannot tolerate conventional MTD chemotherapy, or among those who have already received multiple lines of conventional MTD chemotherapy.
Changing the temporal administration of drugs influences the dynamics of systems involved in a non-linear fashion (14). Thus, the effects of the same nominal dose of a particular drug will be different when administered in a single bolus, versus when administered in multiple small doses. An in-silico simulation has revealed a superiority of metronomic schedule over MTD schedule in terms of reducing disease burden (including metastatic burden). Hence, metronomic chemotherapy is a good approach if desired outcome is of long term control rather than total tumor eradication (14).
While conventional MTD chemotherapy aims solely towards the killing of malignant cells, the metronomic approach owes its efficacy to numerous additional effects. These subtle effects, which are probably masked at routinely used higher doses, include anti-angiogenic effects and immunomodulatory effects. The antiangiogenic effect has been experimentally observed in that metronomic chemotherapy could reduce angiogenic factors such as thrombospondin-1 and vascular endothelial growth factor (VEGF) (15). The immunoregulatory functions are attributable to the increased MHC-1 molecule expression, increased dendritic cell function, and elimination of immunosuppressive factors such as CD4+CD25+ T-regulatory cells, and myeloid derived suppressor cells (16,17).
While reasonable efficacy at exceptional tolerability is a great characteristic of metronomic chemotherapy, it is unfortunately under-utilized clinically even in the palliative setting. As already mentioned, a large proportion of NSCLC patients undergoing palliative RT are unable to tolerate concurrent high dose conventional chemotherapy. Most are either ineligible for or un-affording molecular targeted therapies.
Only two previous studies have been found (in a thorough search involving Medline, Scopus, DOAJ and PubMed Central) which have utilized metronomic chemotherapy during RT for lung cancer, albeit very different from the oral schedule used in our study. Chen et al. in their study involving 70 patients utilized a 3-arm design. One arm received thoracic RT without concurrent chemotherapy, the second arm received thoracic RT with standard dose chemotherapy, and the third arm received metronomic chemotherapy with thoracic RT. About 70% of patients who received metronomic chemotherapy with thoracic RT demonstrated a decrease in plasma VEGF levels. There was no reduction of VEGF in the other two arms (18). Despite the study published in abstract form in 2005, there has unfortunately been no follow-up publication, or any other similar publications in the intervening decade. Another study was a case report by Watanabe et al. describing the successful use of low dose metronomic cyclophosphamide with RT for a single patient of large cell lung carcinoma (19).
The choices of metronomic chemotherapy agents routinely used worldwide include oral daily cyclophosphamide, oral daily etoposide, weekly low dose intravenous cisplatin, thrice-weekly low dose intravenous paclitaxel, among others. However, oral cyclophosphamide was decided upon, owing to the ease of administration (oral), easy availability (as oral cyclophosphamide is ubiquitously available for rheumatological indications), and very easy affordability with 1-month supply of generic oral cyclophosphamide (50 mg tablets) available for as low as INR.300 (equivalent to $5 or ¥30). In contrast, the use of intravenous regimens such as thrice-per-week paclitaxel would cost at-least 100-fold that of oral metronomic cyclophosphamide.
In our study, a very compelling statistically significant enhancement in terms of PFS was observed for patients treated with metronomic oral cyclophosphamide in addition to palliative RT when compared to those treated with palliative RT alone. But surprisingly, this enhancement in PFS (as well as response rates) was strictly limited only for the patients who had the adenocarcinoma histology.
The enhanced outcome for pulmonary adenocarcinoma is a source of optimism for the future. But at the same time, the lack of improvement in outcomes in squamous cell carcinoma histology is rather disappointing. This is a particularly excruciating source of frustration, given that while many forms of specific therapies are available for pulmonary adenocarcinoma (such as pemetrexed, gefitinib, erlotinib, and crizotinib), no forms of specific therapies are available (other than non-specific conventional forms of chemotherapy) for pulmonary squamous cell carcinomas (7). The biological differences between adenocarcinoma and squamous cell carcinoma which lead to a differential response to similar treatment regimens should convince clinicians to abandon the common term ‘NSCLC’. It is very much likely that pulmonary adenocarcinoma and pulmonary squamous cell carcinoma are entirely separate entities in terms of origin, behavior and response to therapies (20,21). This difference must be respected lest regimens appropriate to one histology be incompatible with the other histology.
A larger prospective multi-institutional study is already being considered by us so as to confirm the findings in this study. Further, if selectivity of oral metronomic cyclophosphamide to adenocarcinoma histology alone is confirmed, there will be justification for search of the underlying biological reasons for this specificity. While metronomic cyclophosphamide seems to be ineffective for squamous cell pulmonary carcinoma, it is worthy of evaluation if outcomes in squamous cell carcinoma histology can be improved by the use of other metronomic agents such as low dose tri-weekly intravenous paclitaxel, methotrexate or oral etoposide.
Since our current study has been a retrospective design, there have been a few weaknesses. For example, data regarding overall survival could not be mined from the patient records since most patients prefer to spend their final days at home after progression. The planned prospective trial would be designed in a way as to measure the impact upon overall survival too. Further, a prospective design will also allow us to utilize questionnaires to assess the impact upon quality of life. Univariate and multivariate analyses as per the various prognostic and predictive factors too will be feasible. Very importantly, the use of PERCIST criteria for response evaluation based on positron emission tomography scans (rather than the RECIST criteria which uses CT imaging) will enable better assessment of treatment response (22).
Despite the limitations of a retrospective study, it must however be remarked that our trial presents the first ever data regarding the use of an oral metronomic chemotherapy in conjunction with RT for patients of lung cancer.
Conclusions
Our data indicates that the use of oral metronomic cyclophosphamide at a dose of 50 mg once a day during and after completion of palliative RT improves PFS significantly among patients of pulmonary adenocarcinoma, with no benefits seen in patients of pulmonary squamous cell carcinoma. The findings are compelling enough to justify a larger prospective trial on similar lines. Since a significant proportion of lung cancer patients are eligible only for palliative therapies, this simple approach could hold positive implications. Also, it could be worthwhile to experiment if the combination of oral metronomic cyclophosphamide with oral tyrosine kinase inhibitors could yield benefits. Though it is disappointing that our regimen did not specifically benefit patients with squamous cell carcinoma histology, it could be worthwhile to test agents other than cyclophosphamide for metronomic chemotherapy in this histology.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Weinmann M, Jeremic B, Toomes H, et al. Treatment of lung cancer in the elderly. Part I: non-small cell lung cancer. Lung Cancer 2003;39:233-53. [PubMed]
- Dawe DE, Ellis PM. The treatment of metastatic non-small cell lung cancer in the elderly: an evidence-based approach. Front Oncol 2014;4:178. [PubMed]
- Numico G, Russi E, Merlano M. Best supportive care in non-small cell lung cancer: is there a role for radiotherapy and chemotherapy? Lung Cancer 2001;32:213-26. [PubMed]
- Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247-55. [PubMed]
- Revannasiddaiah S, Thakur P, Bhardwaj B, et al. Pulmonary adenocarcinoma: implications of the recent advances in molecular biology, treatment and the IASLC/ATS/ERS classification. J Thorac Dis 2014;6:S502-25. [PubMed]
- Fojo T, Grady C. How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst 2009;101:1044-8. [PubMed]
- Olszewski AJ, Ali S, Witherby SM. Disparate survival trends in histologic subtypes of metastatic non-small cell lung cancer: a population-based analysis. Am J Cancer Res 2015;5:2229-40. [PubMed]
- Rowell NP, Gleeson FV. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus: a systematic review. Clin Oncol (R Coll Radiol) 2002;14:338-51. [PubMed]
- Faria SL. Role of radiotherapy in metastatic non-small cell lung cancer. Front Oncol 2014;4:229. [PubMed]
- Barroso-Sousa R, da Fonseca LG, Souza KT, et al. Metronomic oral cyclophosphamide plus prednisone in docetaxel-pretreated patients with metastatic castration-resistant prostate cancer. Med Oncol 2015;32:443. [PubMed]
- Revannasiddaiah S, Madabhavi I, Bodh A, et al. Metronomic chemotherapy in anaplastic thyroid carcinoma: a potentially feasible alternative to therapeutic nihilism. Indian J Palliat Care 2015 May-;21:245-9.
- André N, Banavali S, Snihur Y, et al. Has the time come for metronomics in low-income and middle-income countries? Lancet Oncol 2013;14:e239-48. [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [PubMed]
- Benzekry S, Hahnfeldt P. Maximum tolerated dose versus metronomic scheduling in the treatment of metastatic cancers. J Theor Biol 2013;335:235-44. [PubMed]
- Montagna E, Cancello G, Dellapasqua S, et al. Metronomic therapy and breast cancer: a systematic review. Cancer Treat Rev 2014;40:942-50. [PubMed]
- Miller KD, Sweeney CJ, Sledge GW Jr. Redefining the target: chemotherapeutics as antiangiogenics. J Clin Oncol 2001;19:1195-206. [PubMed]
- Hao YB, Yi SY, Ruan J, et al. New insights into metronomic chemotherapy-induced immunoregulation. Cancer Lett 2014;354:220-6. [PubMed]
- Chen Y, Hyrien O, Okunieff P, et al. O-039 Impact of metronomic chemotherapy schedule on circulating VEGF and bFGF during concurrent thoracic radiotherapy. Lung Cancer 2005;49:S16.
- Watanabe Y, Ogo E, Kaida H, et al. Treatment with low-dose cyclophosphamide and radiation therapy for advanced non-small lung cancer in elderly patient. Gan To Kagaku Ryoho 2011;38:1503-5. [PubMed]
- Eilertsen M, Pettersen I, Andersen S, et al. In NSCLC, VEGF-A response to hypoxia may differ between squamous cell and adenocarcinoma histology. Anticancer Res 2012;32:4729-36. [PubMed]
- Pajares MJ, Agorreta J, Larrayoz M, et al. Expression of tumor-derived vascular endothelial growth factor and its receptors is associated with outcome in early squamous cell carcinoma of the lung. J Clin Oncol 2012;30:1129-36. [PubMed]
- Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 2009;50 Suppl 1:122S-50S. [PubMed]


