Metastatic colorectal cancer in a cirrhotic liver with synchronous hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is a leading cause of death globally; in 2012 it was found to be the 5th most common cancer in men and the 9th most common cancer overall (1). The same year it was estimated to be responsible for nearly 745,000 deaths, making it the 2nd most common cause of death from cancer worldwide (2). Colorectal cancer (CRC) is another major cause of morbidity and mortality worldwide, and was found to be the 3rd most common cancer in men and the second in women, but with an overall mortality rate less than that of HCC (3).
Our case report will examine a unique case of a patient developing HCC in the setting of a previous diagnosis (and treatment) of CRC. The situation of developing a second cancer given a previous history of CRC has been found to be relatively high compared to other types of cancers; as the cancer type has the second highest risk of developing subsequent neoplasia; however, the likelihood of developing a metachronous primary cancer is still relatively low at around 8.3% (4).
Since having multiple cancers—collision tumors—is a rare occurrence, reviewing the literature for primary HCC in the setting of metastatic CRC, produced a small number of case reports. In this case, we note a patient who presented with liver metastasis from colorectal adenocarcinoma with coinciding primary HCC in the setting of severe liver cirrhosis secondary to chronic HCV infection.
Case presentation
We present a case of a 59-year-old male with history of stage of 4B colon cancer with metastases to the liver and portal vein infiltration; along with concurrent HCC developed on a background of cirrhosis of the liver with Child Pugh score C, and hepatitis C.
The patient was diagnosed with colon cancer in 2011 and had a sigmoid colectomy in 2012. He received nine cycles of adjuvant chemotherapy with Folfox (5-fluorouracil, leucovorin, and oxaliplatin) in 2012. A repeat colonoscopy in 7/2012 was significant for tubular adenoma without evidence of malignancy. A liver lesion was found on CT abdomen in 03/2014 and FNA in 7/2014 was positive for metastatic adenocarcinoma of the colon. A measured AFP showed an increase from 1,790 in July to 3,640 in August. A CT of the abdomen in August indicated an increasing liver mass, now infiltrating the portal vein. He was started on Folfox and bevacizumab in 9/2014 and received five cycles, with the last one in January 2015.
In February, during a follow up clinic appointment, an MRI of the abdomen showed interval progression of metastatic disease within the liver, with multiple confluent metastases involving the majority of the right hepatic lobe involving segments 5, 6, 7, and 8. One of the lesions in hepatic segment 6 measured 5.6 cm ×5.3 cm; along with extension of tumor into the right portal vein, main portal vein, proximal left portal vein, and middle hepatic vein. Indeterminate HCC vs. metastases in the liver on the MRI were also noted. His AFP was also dramatically elevated at >48,000 with a low CEA of 5.6, which along with the history of cirrhosis, a Child Pugh score of C, and with a hepatitis C diagnosis, was indicative of a new diagnosis of HCC per the consensus report published by the American Association for the Study of Liver Diseases (5).
He was admitted a week later after presenting with epigastric pain. Due to the fact that his HCC had invaded the vessels and one of the lesions was >5 cm he was not a candidate for transplant per the Milan criteria (6). As the patient was not a candidate for chemoembolization due to infiltration of the tumor into the portal vein and as the patient had a poor performance status, sorafenib was deemed not helpful in his case. After a discussion with the patient and the family, he decided to be managed through hospice care.
Imaging
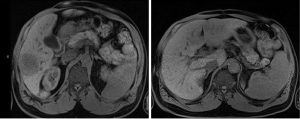
Figure 1 shows an MRI of the abdomen (03/2014) at levels T1 and T2 reveals a hypointensity indicative of HCC with relative absence of enhancement concerning for metastatic adenocarcinoma of the colon to right lobe of the liver, which exhibits no portal venous involvement.
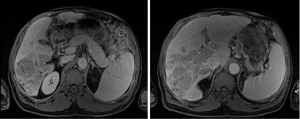
In Figure 2, we see an MRI of the abdomen (02/2015) at levels T1 and T2 revealing hypointensities of infiltrative lesion involving majority of the right hepatic lobe with extension into the portal vein, main portal vein, proximal left portal vein and middle hepatic vein. There is an inability to determine metastases vs. HCC per MRI of the liver.
Pathology
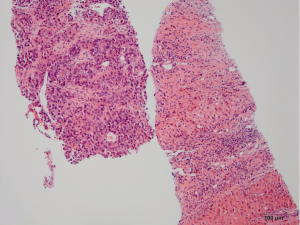
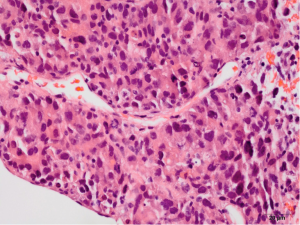
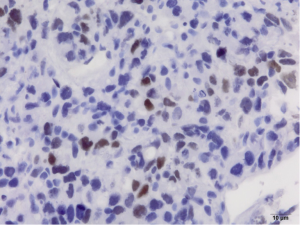
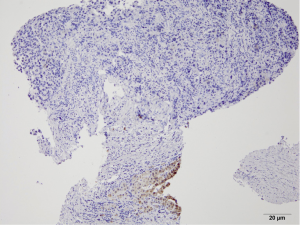
Pathology reveals adenocarcinoma of the colon metastasized to the liver. Of note is the cirrhotic liver parenchyma adjacent to the metastatic carcinoma (HE, 10×) in Figure 3. Higher magnification (40×) in Figure 4 shows malignant cells with high nuclear to cytoplasmic ratio, large hyperchromatic nuclei and occasional prominent nucleoli. Positive nuclear immunohistochemical staining for CDX2 seen in Figure 5 supports the colon origin of this carcinoma. HEPAR immunostain seen in Figure 6 underlies the abnormal architecture of the liver parenchyma, while the metastatic carcinoma is negative for this immunostain.
Discussion
We report a patient with simultaneous colorectal metastases in the liver diagnosed by biopsy, HCC diagnosed on the basis of an aggravation of his pre-existing cirrhosis and hepatitis C diagnosis, with a recently documented dramatic elevation of AFP levels. This is notable because there are exceedingly few cases regarding synchronous colorectal liver metastases and HCC presented in the literature. First, we will discuss the standard approach to treatment of colorectal liver metastases and then the current treatment of HCC; we will then follow this with a discussion of the treatment plan in our patient.
Currently, the only curative approach to colorectal liver metastases is surgical resection. Few patients with liver cirrhosis who develop CRC metastases in the liver are referred for surgical evaluation, mainly because of the important weight of their hepatic co-morbidity. However, oncological surgery in patients with cirrhosis can be performed with a reported mortality rate of less than 5%. A detailed pre-operative assessment should be undertaken, which includes the evaluation of liver function, the identification of portal hypertension, and the measurement of future liver remnant volume and the grade of cholestasis. Surgical treatment in the form of minor hepatectomy can then be performed in highly-selected patients. In patients who are not candidates for surgical resection, interventional radiologic techniques, and locoregional ablative therapies such as radiofrequency ablation (RFA) can be considered with a curative intent if appropriate (7).
There are several options for the treatment of HCC and physicians have used no single algorithm as a reference. In general, it is an accepted theory that surgical resection should be reserved for selected patients in whom the cancer is confined to the liver without evidence of either vascular invasion or portal hypertension, and with an adequate hepatic reserve. Liver transplant is an option in patients who are not candidates for surgical resection due to advanced liver disease and thus poor liver function as opposed to the size of the tumor itself. Orthotropic liver transplant is a suitable option for patients who present with a lesion equal or smaller than 5 cm, or 3 lesions none of which is larger than 3cm, with no distant metastases and no vascular infiltration. Radiofrequency can be considered in patients who do not meet criteria for resection and who demonstrate liver-only disease. TACE is reserved for the cases of large tumors that cannot be treated by RFA. Sorafenib, a multi-targeted tyrosine kinase inhibitor, is the standard systemic treatment of advanced HCC in patients demonstrating good performance status (8). Patients with a poor performance status score are not suitable for this therapeutic approach and are generally managed by supportive care (9).
The case we discussed above denotes the possibility of the presence of two primary cancers. Synchronous HCC and CRC metastatic liver disease is an unusual entity because of the low occurrence of secondary CRC lesions in cirrhotic livers. Determining the appropriate course of treatment was challenging, as the patient was classified as having Child-Pugh C liver disease. Surgical treatment might have been considered, but due to the stage of chronic underlying liver disease in this patient it was not possible. TACE and sorafenib were two other therapeutic approaches that were discussed; however, due to the patient’s poor performance status and declining clinical state, none of the aforementioned treatments were recommended and hospice care was judged to be the best approach in his management.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mustafa GM, Larry D, Petersen JR, et al. Targeted proteomics for biomarker discovery and validation of hepatocellular carcinoma in hepatitis C infected patients. World J Hepatol 2015;7:1312-24. [PubMed]
- Miamen AG, Dong H, Roberts LR. Immunotherapeutic approaches to hepatocellular carcinoma treatment. Liver Cancer 2012;1:226-37. [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [PubMed]
- Ueno M, Muto T, Oya M, et al. Multiple primary cancer: an experience at the Cancer Institute Hospital with special reference to colorectal cancer. Int J Clin Oncol 2003;8:162-7. [PubMed]
- Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [PubMed]
- Ramia JM, López-Andujar R, Torras J, et al. Multicentre study of liver metastases from colorectal cancer in pathological livers. HPB (Oxford) 2011;13:320-3. [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Abdalla EK, Stuart KE. Overview of treatment approaches for hepatocellular carcinoma. Available online: http://www.uptodate.com/contents/overview-of-treatment-approaches-for-hepatocellular-carcinoma?source=search_result&search=hepatocellular+carcinoma+treatment&selectedTitle=1~15s


