Circulating CD40 autoantibody and suPAR synergy drives glomerular injury
Introduction
Focal segmental glomerulosclerosis (FSGS) remains among the leading causes of end-stage kidney disease in children and adults, and has the highest rate of recurrence of all glomerular diseases after kidney transplantation, with florid proteinuria and accelerated renal allograft loss (1). FSGS is a heterogeneous disease with a spectrum of phenotypes based on the existence of recurrent (rFSGS) and non-recurrent (nrFSGS) disease after transplantation, which cannot be easily predicted on disease histopathology (2), and differential disease responses to treatment i.e., immunosuppression (3), plasmapheresis (4), anti-CD20 antibody, and intravenous immunoglobulin (5) in the native and post-transplant states. In addition, infection, drug use, and secondary maladaptive responses after loss of nephrons from any cause may also cause FSGS. Various gene mutations (6) and circulating factors such as suPAR (7) and autoantibodies to CD40 and other glomerular antigens (8) have been associated with FSGS pathogenesis.
CD40 is most commonly known as a co-stimulatory molecule on antigen presenting cells. The significance of CD40 expression variations on podocytes is currently unknown. The mode of CD40 activation and the cell type may dictate cell fate; the binding of CD40 on podocytes by anti-CD40 autoantibodies (CD40 autoAb) in rFSGS, but not in nrFSGS patients induces apoptosis, suggesting a variation in the CD40 autoAb or a difference in signaling, or both. In addition, recent data from our group (8) suggests a synergism of the CD40 autoAb and suPAR pathways in podocytopathy in mice in vivo, as evidenced by heavy proteinuria when the two circulating factors are infused sequentially, this proteinuria effect was minimal/absent when each of them was infused alone. In this study we have repeated the experiment of co-treating wild type C57BL/6 mice with both the CD40 autoAb isolated form the sera of rFSGS patients and human suPAR, to replicate the proteinuria injury previously published by Delville et al. (8) and additionally have resected and examined renal histopathology in these mice at both light microscope and electron microscope levels.
Examination of the pathogenicity of CD40 autoAb and full length suPAR in vivo
To examine the effect of CD40 autoAb and full length suPAR on renal injury, we used female C57BL/6 mice, age 10 weeks, with body weight ranging from 18 to 20 g. Eight and seven mice were randomly chosen to receive intravenous (i.v.) injections of CD40 autoAb isolated from patients with rFSGS, nrFSGS respectively. The dose was inferred from the ratio of CD40 autoAb level in rFSGS patients to that in nrFSGS patients, which is about 4:1. The estimated final concentration was 8 μg/mL for CD40 autoAb from rFSGS and 2 μg/mL for nrFSGS. CD40 autoAb injection was given every other day, for a total of 6 doses. Six hours after the last dose of CD40 autoAb, recombinant human full length suPAR (R&D) protein was given i.v. at 5 μg/mL to all mice. Twenty four hours after the last dose of CD40 autoAb, mouse monoclonal CD40 blocking antibody (Santa Cruz Biotechnology, Inc., Catalog no. sc-59047, Texas, USA) was administered i.p. at a dose of 3 μg per mouse. Urine was collected before and every day after the first injection of CD40 autoAb to analyze urinary albumin with a mouse albumin ELISA kit (Bethyl Laboratories, Inc., Texas, USA) and creatinine (Cayman Chemical Company, Michigan, USA) concentration. Proteinuria is expressed as albumin (mg)/creatinine (g) ratio.
Histological analysis
For histological analysis, the mice were sacrificed with the kidneys excised and fixed in 4% PFA overnight. Periodic acid-Schiff (PAS) staining was performed on paraffin-embedded tissue sections following a standard protocol. In brief, the kidney tissue sections were first deparaffinized via the following steps: 3×5 min Histoclear (Life Science Products Inc., Frederick, Colorado, USA), 3×3 min 100% ethanol, 2×2 min 96% ethanol, 1×1 min 70% ethanol, briefly in distilled water. The deparaffinized sections were then oxidized in 0.5% periodic acid for 10 min, washed 3×5 min with distilled water, and incubated in Schiff’s reagent for 20 min. The differentiation was carried out in sulfite water (12 mL 10% Na2SO3, 10 mL 1M HCl, 200 mL distilled water) 3× for 2 min and a 10 min wash under running tap water. The sections were dehydrated in following steps: 2× briefly 96%, 3×2 min 100% ethanol, 3×2 min Histoclear, and covered with Histokitt. The PAS staining was documented using a Leica DMLB microscope with a Leica DFC425C camera (Leica, Wetzlar, Germany).
Scanning and transmission electron microscopy (TEM)
For scanning electron microscopy (SEM), renal tissues were dissected and sliced thinly into 2 mm pieces. The tissues were fixed in 4% PFA overnight, washed 3× in PBS, dehydrated, critically point dried using the 850 critical point dryer (EMS, Hatfield, Pennsylvenia, USA) and sputter coated in gold on the 108 auto sputter coater (Cressington, Watford, England). For transmission electron microscopy (TEM), renal tissues were collected and dissected into 2-3 mm pieces. The tissues were fixed in 4% PFA O/N, washed 3× in 0.1 mol/L cacodylate buffer and post fixed in 1% OsO4 for 1 h. Tissues were once again washed, dehydrated and embedded in Epon 812. Ultrathin kidney sections (70 nm) obtained on the EM UC7 Ultramicrotome (Leica) were mounted onto Formvar coated Ni slot grids (EMS). Grids were stained for 15 min in 5% uranyl acetate followed by 0.1% lead citrate for 5 min. Electron microscopy micrographs were obtained using the Sigma HDVP Electron Microscope (Zeiss).
Results
CD40 autoAb and full length suPAR synergy induces podocyte injuries
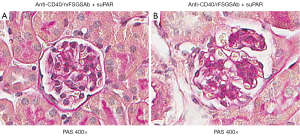
We recently reported that co-administration of CD40 autoAb derived from rFSGS patients (anti-CD40/rFSGSAb) and full length human suPAR into wild type C57BL/6 mice generates significant amount of proteinuria, compared to mice received anti-CD40/nrFSGSAb-suPAR duo. Moreover, the levels of proteinuria induced by anti-CD40/rFSGSAb-suPAR duo are much higher than that caused by either anti-CD40/rFSGSAb or full length suPAR alone (8). To examine this rise in proteinuria associated kidney alteration, we performed PAS staining. As shown in Figure 1, the kidney morphology was largely unaltered in either anti-CD40/rFSGSAb-suPAR or anti-CD40/nrFSGSAb-suPAR dually treated mice. However, focal glomerular hypercellularity and hyalinosis was observed in some of the glomeruli in anti-CD40/rFSGSAb-suPAR duo treated mice.
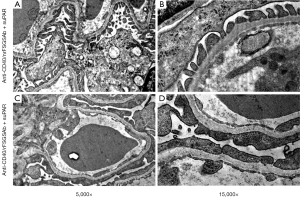
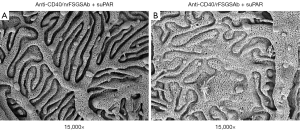
To examine the impact of anti-CD40/rFSGSAb-suPAR on glomerular ultrastructure, we performed TEM and SEM analysis. Under TEM, significant podocyte foot process effacement was observed in some of the glomeruli in anti-CD40/rFSGSAb-suPAR duo injected mice (Figure 2A,B); whereas, this phenomenon was not detected in anti-CD40/nrFSGSAb-suPAR treated mice (Figure 2C,D). Under SEM, while podocyte foot processes interdigitating with each other is observed in the glomeruli from anti-CD40/nrFSGSAb-suPAR treated mice (Figure 3A); podocyte process fusion and/or flattening is readily seen in some glomeruli of the kidney from anti-CD40/rFSGSAb-suPAR treated mice (Figure 3B). These data put together, indicate that CD40 autoAb derived from rFSGS patients and full length suPAR dual treatment induce podocyte injuries and proteinuria in wild type mice.
Discussion
This study further supports earlier observation that CD40 autoAb isolated form the sera of patients with rFSGS synergize with full length suPAR in mice to produce extensive foot process effacement, heavy proteinuria and histological evidence of glomerular sclerosis. Only minimal proteinuria was observed with multiple separate injection of either human CD40 autoAb from rFSGS patients or human full length suPAR alone, suggesting an additive role of these two circulating factors in the etiopathogenesis of podocytopathy in FSGS disease. The pathogenic human CD40 autoAb appears to be an important priming trigger for increasing the sensitivity of the glomerulus for co-existing nephrotoxic factors such as human suPAR. Interestingly, Alfano et al. have recently shown that full length suPAR infusion causes nephrin internalization and proteinuria in mice absent urokinase receptor but no immediate proteinuria was found in wildtype mice (9), a notion which was already reported earlier by Wei et al. (7). Wei et al. further showed that blood circulation of an alternative transcripted suPAR (consisting of D1 and part of D2) has strong pathogenicity for wildtype mice. We have not tested this variant yet in combination with pathogenic human CD40 autoAb. Clearly, the roles of suPAR forms in concert with CD40 autoAb have to be elucidated in particular for rFSGS as the disease phenotype occurs fast and with severe podocyte injury.
In addition, we need to define how pathogenic human CD40 autoAb and suPAR interact and if they act in the same pathway such as on β3 integrin or if there are other pathways involved.
Acknowledgements
We would like to thank Jing Li and Nicolas Tardi (Rush University) for technical help.
Footnote
Conflicts of Interest: MM Sarwal is the Founder of Organ-I Inc. and has issued patents for transplant biomarkers. MM Sarwal is on the Scientific Advisory Boards for Organ-i Inc. and Immucor and is/was a consultant for Bristol-Myers Squibb, Novartis, Isis, Immucor, Roche and Genentech. J Reiser has pending or issued patents on antiproteinuric therapies, has served as an adviser for the Abbott Renal Scientific Advisory Council, Genzyme Renal Innovations Program, Johnson & Johnson Renal Program, Genentech, and Questcor, and is or was a paid consultant for Johnson & Johnson, Abbott, Amgen, AstraZeneca, Genentech, Genzyme, Merck KG, Merck, Pfizer, Questcor, Roche, ViroGates, and Trisaq. C Wei has a pending patent on novel biomarkers for diabetic kidney disease.
References
- Verani RR, Hawkins EP. Recurrent focal segmental glomerulosclerosis. A pathological study of the early lesion. Am J Nephrol 1986;6:263-70. [PubMed]
- Canaud G, Dion D, Zuber J, Gubler MC, et al. Recurrence of nephrotic syndrome after transplantation in a mixed population of children and adults: course of glomerular lesions and value of the Columbia classification of histological variants of focal and segmental glomerulosclerosis (FSGS). Nephrol Dial Transplant 2010;25:1321-8. [PubMed]
- Gallon L, Leventhal J, Skaro A, et al. Resolution of recurrent focal segmental glomerulosclerosis after retransplantation. N Engl J Med 2012;366:1648-9. [PubMed]
- Straatmann C, Kallash M, Killackey M, et al. Success with plasmapheresis treatment for recurrent focal segmental glomerulosclerosis in pediatric renal transplant recipients. Pediatr Transplant 2014;18:29-34. [PubMed]
- Kronbichler A, Kerschbaum J, Fernandez-Fresnedo G, et al. Rituximab treatment for relapsing minimal change disease and focal segmental glomerulosclerosis: a systematic review. Am J Nephrol 2014;39:322-30. [PubMed]
- Pollak MR. The genetic basis of FSGS and steroid-resistant nephrosis. Semin Nephrol 2003;23:141-6. [PubMed]
- Wei C, El Hindi S, Li J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 2011;17:952-60. [PubMed]
- Delville M, Sigdel TK, Wei C, et al. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med 2014;6:256ra136.
- Alfano M, Cinque P, Giusti G, et al. Full-length soluble urokinase plasminogen activator receptor down-modulates nephrin expression in podocytes. Sci Rep 2015;5:13647. [PubMed]


