Determinants of the incidence of Duchenne muscular dystrophy
Introduction
Yiu and Kornberg review the clinical features, investigations and management, including novel therapies, of Duchenne muscular dystrophy (DMD) (1). They note that it is an X-linked disorder and the most common muscular dystrophy with an incidence of one in 5,000 boys, which are 200 per million births. It presents in early childhood with death in late teens.
Cowan et al. found that the overall incidence of DMD in New South Wales and the Australian Capital Territory for the period 1960-1971 was 186 per million, from 99 cases (2). They found that about 14% of the cases were ‘theoretically preventable’. An affected male with at least one affected male relative in a previous generation in the female line was classified as theoretically preventable, as was an affected brother born at least 6 years after an index case.
Moat et al. report the screening of newborn bloodspots which was introduced in Wales in 1990 (3). The incidence of DMD during the screening period was 1:5,136 (195 per million), compared with 1:4,046 (247 per million) before commencement of screening.
In an editorial in The Journal of Pediatrics published in 2009, Kaufmann noted that, despite all the advances made up to that time, “there has been no significant change over the past 20 years in the time from symptom onset to diagnosis” (4). Kaufmann’s editorial was prompted by the article published in the same issue of the journal by Ciafaloni et al. who stated: “The purpose of this population-based investigation was to describe the timeline in the diagnostic process among individuals without known family history of DMD, to identify reasons for delays in diagnosis, and to highlight clinical steps needed to shorten the time to diagnosis.” At this time the authors were still recommending: “checking creatine kinase early in the evaluation of boys with unexplained developmental delay” (5).
Tuan-Pham et al. and Nguyen et al. present material which foreshadow that the incidence is likely to be reduced due to recent findings about carrier recognition and genetic counselling (6,7). Tuan-Pham et al. foresee “enabling embryo transfer from the preimplantation genetic diagnosis (PGD) cycle” (7). The following section of this paper includes provision for the likely effects of intervention.
The third section summarises some findings from attempts to estimate the mutation rates in eggs and sperm and the proportion of cases arising from mutation in the eggs of non-carrier mothers and their relation to the model given in the next section.
Population genetics of DMD
The following is a deterministic model which attempts to relate the incidence of DMD to the mutation rate in eggs, denoted by µE, and sperm µS. Denote the frequency of the ‘normal’ gene in the population by q, so that the frequency of the gene on the X-chromosome which causes DMD in boys is 1–q. Assume that the frequency of normal homozygous females is x=q2 and that of carrier females is h =2q(1–q). We denote the frequency of normal males by y=q.
Assuming discrete, non-overlapping generations, the incidence of DMD in boys is

In the following generation, the frequencies are

and

In this generation the frequency of the normal gene in females is

and in (normal) males is

We define the frequency of the normal gene in the population as

Assuming values of µE and µS, we use the above formulae to generate a sequence of generations, including, in particular, the incidence of DMD. After several generations the equilibrium gene frequency is reached, together with the incidence of DMD from Eq. [1].
As a refinement, we modify Eq. [1] to allow for the possibility that female carriers of the DMD gene are identified leading to a reduction in incidence. The modified incidence is given by

In Eq. [7], r is the coefficient of carrier recognition. When r=1, no cases arise by transmission from existing carriers, so that the incidence is xµE. The mutation rates are small, of the order of 10−5, so that, when r=1, x =1, the incidence of DMD is approximately equal to the mutation rate in eggs. We rely on such approximations in what follows.
Using the results of iteration runs using the above formulae, the frequency of the DMD gene in equilibrium is approximately

The incidence of DMD is
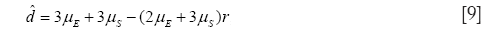
so that, as noted above, when r=1, the incidence of DMD is equal to the mutation rate in eggs.
Male to female ratio of mutation rates
Müller and Grimm give a method of estimating the relative mutation rates µS / µE (8). In their introduction they reproduce a formula given by J. B. S. Haldane in 1935:

where U is the mean mutation rate, f the effective fertility of affected males and I the incidence of the disease. The correspondence with our notation is: U = µE + µS, whereas Haldane’s definition is U = (2µE + µS)/3; I=d; f=0. This leads to U = I/3 and so is equivalent to Eq. [9], providing that r=0. These authors note that several studies have reported U = 7-10 × 10-5, which is “much in excess of any other mutation rate in man”. In their discussion they write: “Classical methods for the estimation of mutation rates in man indicate an almost equal rate in both sexes for the Duchenne mutation. This is in clear contrast to the findings in other well studied X-linked disorders.”
Substituting µE = µS =7×10−5 and r = 0.55 in Eq. [7] gives incidence of affected males 227 per million births of whom about 31% are produced from a mutation in the egg of (non-carrier) mothers. The percentage is obtained from Eq. [7] as 100× xµE /d, after using Eq. [8] to calculate the gene frequencies and the other terms from identities given above. This demonstrates how it is possible to produce rates consistent with observed counts by manipulating the input parameters.
Nguyen et al. state: “The rate of mutation is 28.7%” (6). We take it that this measure is the rate of mutation in eggs of non-carrier mothers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Yiu EM, Kornberg AJ. Duchenne muscular dystrophy. J Paediatr Child Health 2015;51:759-64. [PubMed]
- Cowan J, Macdessi J, Stark A, et al. Incidence of Duchenne muscular dystrophy in New South Wales and Australian Capital Territory. J Med Genet 1980;17:245-9. [PubMed]
- Moat SJ, Bradley DM, Salmon R, et al. Newborn bloodspot screening for Duchenne muscular dystrophy: 21 years experience in Wales (UK). Eur J Hum Genet 2013;21:1049-53. [PubMed]
- Kaufmann P. Missed opportunities for duchenne muscular dystrophy. J Pediatr 2009;155:309-10. [PubMed]
- Ciafaloni E, Fox DJ, Pandya S, et al. Delayed diagnosis in duchenne muscular dystrophy: data from the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet). J Pediatr 2009;155:380-5. [PubMed]
- Nguyen TM, Tien NM, Nguyen TP, et al. Molecular diagnosis outcome of Duchenne muscular dystrophy gene after 10 years in Vietnam. Ann Transl Med 2015;3:AB090.
- Tuan-Pham LA, Tran TH, Tran DQ, et al. Microsatellite markers for preimplantation genetic diagnosis in Vietnamese DMD and hemophilia: a female carriers. Ann Transl Med 2015;3:AB163.
- Müller CR, Grimm T. Estimation of the male to female ratio of mutation rates from the segregation of X-chromosomal DNA haplotypes in Duchenne muscular dystrophy families. Hum Genet 1986;74:181-3. [PubMed]


