Predicting Wolbachia potential to knock down dengue virus transmission
The failure of traditional disease prevention methods to halt the current progression of dengue has promoted the development of novel entomological strategies. One of the most promising approaches relies on the intracellular bacterium Wolbachia, a bacterial symbiont commonly found in arthropods (1). The main mosquito vector of dengue viruses, Aedes aegypti, does not naturally carry Wolbachia, but can be experimentally transinfected by embryonic microinjection (2). Transinfection of Ae. aegypti with certain strains of Wolbachia results in protection against dengue virus infection (3,4). Thus, successful establishment of Wolbachia in natural mosquito populations (5) supports a practical approach for dengue suppression. The next critical step is to assess the epidemiological efficacy of Wolbachia in reducing dengue virus transmission in the field (6).
A recent study by Ferguson and colleagues (7) lays the ground for future efficacy trials by quantitatively predicting the likely impact of Wolbachia on dengue virus transmission. Their study makes two significant advances. First, it provides empirical data on the vector competence of Wolbachia-infected Ae. aegypti using viremic blood from dengue patients and therefore more closely mimics field conditions than earlier studies based on laboratory challenge with cultured virus. Vector competence was evaluated by testing the presence of viral infection in the mosquito abdomen and salivary glands or saliva at different time-points after the infectious blood meal. Second, it develops a mathematical framework to describe the dynamics of dengue virus transmission between humans and mosquitoes. The model is then fitted to the empirical vector competence data to predict the effect of Wolbachia on the basic reproduction number (R0) of dengue virus transmission. R0 is the average number of subsequent infections resulting from an infected human introduced in a naive population. Estimates of R0 for dengue typically range from 2 to 5 (8). A pathogen will go to extinction if R0 is less than one because it means that each infected individual will generate less than one new infection on average.
The study assessed the vector competence of Ae. aegypti mosquitoes carrying one of two Wolbachia strains. The first strain called wMelPop is characterized by high bacterial densities in mosquito tissues and results in almost complete refractoriness to dengue virus infection in laboratory challenge (4). However, it also induces deleterious effects on mosquito fitness such as reduced lifespan and blood feeding success (3,9). Experiments using viremic blood from dengue patients confirmed the strong protective effect of wMelPop against dengue virus, although systemic infection was not completely blocked. Only 2.6% of Wolbachia-infected mosquitoes had virus-positive salivary glands, compared to 90% in Wolbachia-free controls. The authors concluded that wMelPop would result in at least 90% blocking of transmission. The second Wolbachia strain called wMel infects mosquito tissues at lower densities, and induces resistance to dengue virus infection in laboratory challenge, although to a lesser extent than wMelPop, and in the absence of major fitness costs (10). Consistently, there was significant but imperfect virus blocking in mosquitoes infected by wMel challenged with viremic blood from dengue patients. Although viral load measured in the abdomen was at least 10-fold lower in Wolbachia-infected mosquitoes, most of the blocking effect was observed during viral dissemination from the abdomen to the saliva. The effect comprised a net reduction of the probability of saliva infection, and a slight lengthening of the time required for the virus to reach saliva.
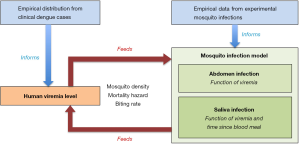
Ferguson et al. (7) then used the empirical data generated in their vector competence assays as well as clinical records of viremia levels in patients to inform a newly developed mathematical model of dengue virus transmission (Figure 1). The model was designed to evaluate the effect of Wolbachia on dengue virus transmission based on the comparison of R0 in a mosquito population with or without Wolbachia. The modeling approach only considered wMel because wMelPop did not require mathematical modeling to predict quasi-complete blocking of transmission. The mosquito infection model consisted of a relatively simple dose-response model of abdomen infection probability as a function of viremia coupled to a model of saliva infection probability as a function of viremia as well as time elapsed since the blood meal (Figure 1). Model fitting to the empirical data was performed separately for each of the four dengue virus serotypes. The baseline scenario predicted 66-75% of reduction in R0 depending on the dengue serotype. Other scenarios were considered to account for the uncertainty in model parameters that were not directly informed by empirical data such as the minimum infectious dose for successful mosquito-to-human transmission. The percentage in R0 reduction varied from 40% to 80% among serotypes under the alternative scenarios. Therefore, under the baseline model, a Wolbachia intervention using the wMel strain is expected to result in two thirds to three quarters less secondary infections from an initial case. This means that the intervention would achieve elimination of dengue for initial R0 values of 3 or 4, respectively. Thus, Ae. aegypti mosquitoes carrying wMel could reduce dengue virus transmission by a degree that would have considerable public health impact, possibly leading to dengue elimination where transmission is low to moderate (8).
A major strength of the Ferguson et al. study (7) is the use of state-of-the-art methods to evaluate vector competence. Historically, methods of determining vector competence have been largely restricted to artificial infectious blood meals composed of animal blood spiked with virus grown in cell culture. These artificial methods have limited our ability to extrapolate to natural transmission and to understand the significance of data from epidemiological studies with humans (11). Recent studies from the same group overcame this obstacle by developing vector competence assays that expose mosquitoes to the blood of naturally infected, viremic humans (12). Although in the present study viremic blood was presented to mosquitoes in an artificial feeder through a skin-simulating membrane, it is reasonable to consider this indirect mosquito feeding method as a good proxy of direct feeding through the skin of a person. Nevertheless, vector competence is only one of several parameters that influence dengue virus transmission by mosquitoes (11). It will be necessary in future studies to evaluate the effect of wMel on several important entomological parameters that Ferguson et al. did not examine in their study such as blood feeding behavior and longevity. For instance, a shorter lifespan could act to further reduce dengue virus transmission. Conversely, increased blood feeding frequency would enhance transmission. The wMelPop strain confers very strong protection against dengue virus infection and further limits transmission by shortening the mosquito lifespan (3,4). But the life-shortening effect would represent a significant hurdle to establishing wMelPop infection in a natural Ae. aegypti population by reducing competitiveness against wild mosquitoes. Overall, the costs and benefits of each Wolbachia strain will have to be carefully balanced prior to field releases.
One limitation of the Ferguson et al. study (7) is that the transmission model relies on a distribution of viral titers in plasma that may not accurately reflect reality, for at least two reasons. First, the empirical distribution of plasma viremia levels that were used to develop the transmission model only included hospitalized and ambulatory patients. This distribution, therefore, did not consider inapparent (subclinical) infections that are believed to represent the majority of dengue infections (13). People with inapparent infections are usually assumed to inefficiently infect mosquitoes because they do not reach sufficiently high viremia levels, but this assumption has not been verified (14). Second, the transmission model did not account for the epidemiological feedback. Put simply, introduction of Wolbachia-infected mosquitoes could affect the distribution of viremia levels in humans, and consequently modify the baseline parameters underlying the model that estimates transmission. The authors considered that modeling three distributions recapitulates the complete transmission cycle (Figure 1): human viremia level, human-to-mosquito transmission probability (abdomen infection), and mosquito-to-human transmission probability (saliva infection). In fact, a parameter characterizing the relationship between mosquito-to-human transmission and the resulting viremia profile is missing from the cycle. One could imagine, for instance, that Wolbachia-infected mosquitoes inoculate smaller infectious doses that result in shorter, shallower viremia profiles. In both cases, fortunately, these shortcomings likely contributed to underestimate the impact of Wolbachia on dengue virus transmission. Indeed, the transmission blocking effect of Wolbachia would be stronger if viremia levels were reduced compared to those seen in dengue-infected people with clinical symptoms.
Taken together, this work and previous studies support the idea that Wolbachia has a realistic potential to knock down dengue virus transmission in the field. It is also clear, however, that Wolbachia alone will not be sufficient to effectively control dengue, especially in settings where transmission is high. In addition to novel vector population suppression strategies (15) and vaccines (16), Wolbachia may soon enrich the arsenal to effectively fight against dengue.
Acknowledgements
L Lambrechts is supported by the Emergence(s) Program in Biomedical Research of the City of Paris and by the French Government’s Investissement d’Avenir program, Laboratoire d’Excellence Integrative Biology of Emerging Infectious Diseases (grant ANR-10-LABX-62-IBEID).
Footnote
Provenance: This is a Guest Editorial commissioned by Executive Editor Bing Gu, MD (Department of Laboratory Medicine, the Affiliated Hospital of Xuzhou Medical University, Xuzhou, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Hilgenboecker K, Hammerstein P, Schlattmann P, et al. How many species are infected with Wolbachia?--A statistical analysis of current data. FEMS Microbiol Lett 2008;281:215-20. [PubMed]
- Xi Z, Khoo CC, Dobson SL. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 2005;310:326-8. [PubMed]
- McMeniman CJ, Lane RV, Cass BN, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 2009;323:141-4. [PubMed]
- Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009;139:1268-78. [PubMed]
- Hoffmann AA, Montgomery BL, Popovici J, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011;476:454-7. [PubMed]
- Lambrechts L, Ferguson NM, Harris E, et al. Assessing the epidemiological effect of wolbachia for dengue control. Lancet Infect Dis 2015;15:862-6. [PubMed]
- Ferguson NM, Kien DT, Clapham H, et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med 2015;7:279ra37.
- Johansson MA, Hombach J, Cummings DA. Models of the impact of dengue vaccines: a review of current research and potential approaches. Vaccine 2011;29:5860-8. [PubMed]
- Turley AP, Moreira LA, O'Neill SL, et al. Wolbachia infection reduces blood-feeding success in the dengue fever mosquito, Aedes aegypti. PLoS Negl Trop Dis 2009;3:e516. [PubMed]
- Walker T, Johnson PH, Moreira LA, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011;476:450-3. [PubMed]
- Lambrechts L, Failloux AB. Vector biology prospects in dengue research. Mem Inst Oswaldo Cruz 2012;107:1080-2. [PubMed]
- Nguyet MN, Duong TH, Trung VT, et al. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A 2013;110:9072-7. [PubMed]
- Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature 2013;496:504-7. [PubMed]
- Carrington LB, Simmons CP. Human to mosquito transmission of dengue viruses. Front Immunol 2014;5:290. [PubMed]
- Carvalho DO, McKemey AR, Garziera L, et al. Suppression of a Field Population of Aedes aegypti in Brazil by Sustained Release of Transgenic Male Mosquitoes. PLoS Negl Trop Dis 2015;9:e0003864. [PubMed]
- Hadinegoro SR, Arredondo-García JL, Capeding MR, et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N Engl J Med 2015;373:1195-206. [PubMed]