Multilayered T-cell memory in human skin
The skin represents a highly complex immunological microenvironment capable of protecting the organism from infectious pathogens. Accordingly, the human skin harbors large quantities of immune cells, including billions of memory T cells (1,2). While some of these T cells are constantly recirculating through the skin and thus are in equilibrium with their counterparts in the blood, others remain permanently resident in the skin and never return to the circulation (2). A recent study published in Science Translational Medicine by Watanabe and colleagues (3) has shed new light on the composition of these recirculating and resident T-cell subsets in human skin, and has linked the malignant transformation of individual subsets to the variable clinical presentation of cutaneous T-cell lymphoma (CTCL).
Memory T cells provide long-lived pathogen-specific immunity in lymphoid and peripheral tissues, including barrier locations such as skin and mucosa. These tissues contain a combination of recirculating and permanently resident memory T (TRM) cells and studies in mice have recently identified the latter (TRM cells) as the central mediators of localized protective immunity (4-9). However, TRM cells may also drive aberrant immune responses that are associated with autoimmunity, transplant rejection and malignancies (10,11). In skin for instance, such pathogenic T-cell responses are observed in psoriasis, alopecia, contact hypersensitivity and CTCL. Consistent with a TRM-cell involvement, such diseases often present with chronic or recurrent lesions in fixed anatomical locations (10-14). While detailed information on the mode of tissue residency, in situ differentiation and protective function of TRM cells in skin has emerged from mouse studies (11,15,16), obvious technical and ethical constraints have thus far limited our knowledge about the relative proportions and effector activities of resident and recirculating T-cell subsets in human skin.
Watanabe and colleagues employed an elegant skin xenograft model to study T-cell dynamics in human skin (3). They transplanted human neonatal foreskin onto immune-deficient mice and, at the same time, transferred allogenic human peripheral blood cells from unrelated donors. The transferred T cells were activated in the skin grafts via allorecognition, which resulted in the induction of a dermatitis and subsequently, in the generation of various populations of graft-resident memory T-cells. A subset of these T cells up-regulated CD69 shortly after migration into the grafts and some of these cells co-expressed the integrin subunit CD103, thereby resembling the TRM cells described in mouse skin (5,6). There were also T cells that displayed a central memory T-cell (TCM) phenotype, as indicated by expression of the lymph node (LN)-targeting migration receptors CCR7 and L-selectin. Importantly, all subsets identified in human-engrafted mice were also detected in healthy adult skin not subjected to tissue transplantation, therefore further emphasizing the validity of the xenograft model.
A previous study by the same group has shown that circulating T cells in CTCL patients can be depleted with low doses of alemtuzumab, a humanized anti-CD52 antibody, while skin TRM cells remain unaffected by this treatment (17). In the present study, Watanabe and colleagues found that the same was true for human-engrafted mice. Alemtuzumab treatment lead to depletion of T cells from the blood but spared some of the T-cell subsets in the graft, making it possible to discriminate between recirculating and skin-resident memory T cells. Analysis of remaining and thus non-recirculating TRM cells in alemtuzumab treated human-engrafted mice and CTCL patients revealed that virtually all of them expressed CD69. This is consistent with studies in mice, where TRM cells commonly express CD69 (15,16). This molecule has been shown to promote prolonged effector T-cell retention in skin by interfering with the tissue exit receptor, sphingosine-1-phosphate receptor 1 (18). A fraction of both CD4+ helper and CD8+ killer T cells remaining in the grafts for at least 3 weeks of alemtuzumab treatment also expressed CD103, the α-subunit of the αEβ7 integrin thought to mediate tethering of TRM cells to their microenvironment by binding to epithelial cells expressing the CD103 ligand, E-cadherin (15). Interestingly, CD103+ TRM cells were enriched in the epidermal layer, although considerable populations of helper and killer CD103+ cells were also detected in the dermis. Given that a similar epidermotropism of CD103+ TRM cells has also been described in mice (5,19,20), it is tempting to speculate that the dermal CD103+ cells may preferentially associate with skin appendages of epithelial origin, such as glands or hair follicles, and/or represent specialized subsets such as regulatory T cells, of which some express CD103 in human skin (21). Functional assays on isolated T cells further revealed that both CD103+ and CD103− TRM subsets exhibited a heightened capacity to produce effector cytokines, but had lower proliferative potential when compared to their recirculating CD69− counterparts. This is an important piece of data fitting well with the overall concept that TRM cells provide superior local immune defense.
Although not readily replicated in the xenotransplant model, the authors further identified a forth T-cell subset in the skin of healthy individuals and CTCL patients. This additional subset, termed migratory memory T cells (TMM), expressed CCR7 but not L-selectin and therefore, differed from the CCR7+ L-selectin+ TCM cells. Remarkably, these TMM cells were the most abundant population amongst skin-tropic T cells in the blood of healthy individuals, as identified by their expression of the skin homing molecule, cutaneous leukocyte antigen. Importantly, TMM cells were depleted in both the circulation and the skin of CTCL patients treated with alemtuzumab, although depletion from skin was much slower compared to that of TCM cells. Thus, TMM cells represented a recirculating T-cell subset with distinct migration kinetics. Furthermore, given that these cells lacked expression of L-selectin and therefore should be excluded from entering LNs from the blood, the authors speculated that TMM cells might also have a specialized recirculation pattern. In support of this hypothesis, Watanabe and colleagues reported an intriguing link between the TCM/TMM phenotypes of malignant skin-tropic T-cell clones and the varying clinical pictures in CTCL patients.
CTCL can present as skin-limited forms, such as mycosis fungoides, or leukemic forms, including the Sézary syndrome (22). Patients with early stage mycosis fungoides have fixed skin lesions and transformed T-cell clones are usually absent from LNs and blood. By contrast, leukemic forms of CTCL present with more disseminated lesions with ill-defined borders that in their most extreme form can result in generalized erythroderma. The malignant T-cell clones in these patients are usually found in the blood and lymphoid tissues, including skin-draining as well as systemic LNs. Elegant earlier work by Clark and Kupper and colleagues has demonstrated that the leukemic CTCL forms are caused by recirculating skin-homing memory T cells, which can be successfully depleted by alemtuzumab treatment (17,23). Mycosis fungoides on the other hand originates from malignant TRM cells that are unresponsive towards the same treatment (17,23). In the current study, Watanabe and colleagues analyzed malignant T-cell clones in leukemic CTCL patients with different clinical manifestations and found a pattern consistent with the proposed differential migration of TCM and TMM cells. Patients with malignant CCR7+ L-selectin+ TCM cells showed diffuse skin lesions with local as well as systemic LN involvement. By contrast, patients harboring malignant clones with a predominantly CCR7+ L-selectin− TMM phenotype displayed more discrete skin lesions, albeit with ill-defined borders, and had involvement of skin-draining, but not systemic LN. Thus, these clinical data are consistent with a scenario where TMM cells migrate between the skin and blood via the lymphatics and skin-draining LNs. Importantly, however, the TMM cells are excluded from entering lymphoid tissues draining other organs due to their lack of L-selectin expression and their inability to enter LN from the blood. Conversely, TCM cells similarly recirculate between skin and blood but also access remote LNs via high endothelial venules in a L-selectin and CCR7-depedendent manner (Figure 1). Future studies will have to further validate this concept of specialized migratory memory T cells for other organ systems and will have to investigate the contribution of skin-tropic TCM and TMM cells to other types of skin diseases.
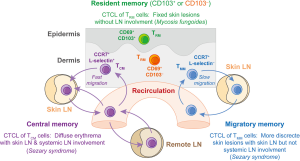
Taken together, Watanabe and colleagues have made an important contribution to our understanding of T-cell responses in human skin. Careful interpretation of clinical data in combination with the use of sophisticated xenograft models allowed the authors to identify four distinct populations of skin T cells with vastly different migration patterns and effector potential (Figure 1). As well as extending our basic concepts of T-cell immune surveillance in skin, this work also highlights the predictive value that the identification of the various T-cell subsets has for therapy outcomes in patients with various forms of Sézary syndrome. Likewise, determining the contribution of these distinct memory subsets to the pathogenesis of other diseases may improve future treatments in T-cell mediated inflammatory skin conditions, such as psoriasis, vitiligo, alopecia or contact hypersensitivity. Finally, understanding the precise roles recirculating and resident T cells play in immune defense against infectious diseases will be critical for the development of novel vaccination strategies that aim to protect the body’s surfaces in skin and mucosa.
Acknowledgements
The authors are supported by fellowship and grant funding from the National Health and Medical Research Council in Australia, the German Research Foundation and the University of Melbourne, respectively.
Footnote
Provenance: This is a Guest Editorial commissioned by Executive Editor Bing Gu, MD (Department of Laboratory Medicine, the Affiliated Hospital of Xuzhou Medical University, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Clark RA, Chong B, Mirchandani N, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol 2006;176:4431-9. [PubMed]
- Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat Immunol 2013;14:978-85. [PubMed]
- Watanabe R, Gehad A, Yang C, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med 2015;7:279ra39.
- Ariotti S, Hogenbirk MA, Dijkgraaf FE, et al. T cell memory. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science 2014;346:101-5. [PubMed]
- Gebhardt T, Wakim LM, Eidsmo L, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 2009;10:524-30. [PubMed]
- Jiang X, Clark RA, Liu L, et al. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 2012;483:227-31. [PubMed]
- Mackay LK, Stock AT, Ma JZ, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A 2012;109:7037-42. [PubMed]
- Schenkel JM, Fraser KA, Beura LK, et al. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 2014;346:98-101. [PubMed]
- Bromley SK, Yan S, Tomura M, et al. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol 2013;190:970-6. [PubMed]
- Clark RA. Resident memory T cells in human health and disease. Sci Transl Med 2015;7:269rv1.
- Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 2015;21:688-97. [PubMed]
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med 2014;20:1043-9. [PubMed]
- Conrad C, Boyman O, Tonel G, et al. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med 2007;13:836-42. [PubMed]
- Gaide O, Emerson RO, Jiang X, et al. Common clonal origin of central and resident memory T cells following skin immunization. Nat Med 2015;21:647-53. [PubMed]
- Gebhardt T, Mackay LK. Local immunity by tissue-resident CD8(+) memory T cells. Front Immunol 2012;3:340. [PubMed]
- Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity 2014;41:886-97. [PubMed]
- Clark RA, Watanabe R, Teague JE, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med 2012;4:117ra7.
- Mackay LK, Braun A, Macleod BL, et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol 2015;194:2059-63. [PubMed]
- Ariotti S, Beltman JB, Chodaczek G, et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci U S A 2012;109:19739-44. [PubMed]
- Gebhardt T, Whitney PG, Zaid A, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 2011;477:216-9. [PubMed]
- Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood 2007;109:194-202. [PubMed]
- Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet 2008;371:945-57. [PubMed]
- Campbell JJ, Clark RA, Watanabe R, et al. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood 2010;116:767-71. [PubMed]


