
Protective effect of ischemic postconditioning on ischemia reperfusion injury in steatotic rat livers
Introduction
Ischemia/reperfusion (I/R) injury is a significant factor in liver damage that occurs during hepatic inflow occlusion reperfusion via portal triad clamping (the Pringle maneuver) after a hepatectomy and also after a liver transplantation (1). Hepatic I/R injury could result in local and remote multi-organ dysfunction (2), and unfortunately, is inevitable in many situations. Steatotic livers are more susceptible to I/R injury than normal livers (3). Multiple studies have reported that steatosis is a significant risk factor for postoperative liver failure after a hepatectomy, and a causative factor for primary graft incompetence or dysfunction during liver transplantation (4-7). Additionally, hepatic steatosis is the most common metabolic disorder with an incidence of 20–30% in Western countries (8). Due to the increasing number of orthotopic liver transplantations and the scarcity of fit donor livers, many transplant centers are using “marginal” livers, such as steatotic livers, in liver transplantations (6). These realities emphasize the necessity of steatotic liver protection during liver surgery or transplantation.
It has been hypothesized that steatotic livers have an increased susceptibility to I/R injury. The hypothesis may manifest as lipid peroxidation, neutrophil infiltration, microcirculatory alterations, decreased intracellular energy levels, and the release of proinflammatory mediators [e.g., tumor necrosis factor (TNF)-α] (1,9,10). However, the specific roles of these different mechanisms have not yet been elucidated.
Previous research has shown that ischemic preconditioning (IPreC) is an endogenous process that protects multiple organs from I/R injury, including the normal liver, through transient vascular occlusion before sustained ischemia (11-14). Additionally, IPreC has been shown to protect steatotic livers from I/R injury in the course of warm ischemia and transplantation (15,16). The underlying mechanisms of IPreC are not yet known. IPreC protects against I/R injury; however, its use as a clinical strategy is greatly limited by the fact that it is sometimes difficult to implement before I/R injury occurs (17). Ischemic postconditioning (IPostC), defined as several brief cycles of ischemia and reperfusion performed immediately in the initial reperfusion period after ischemia, has been proven to increase several organs’ ability to tolerate I/R injury (11,18-21). A previous study has indicated that IPostC can suppress the apoptosis of liver cells and reduce reperfusion injury of liver tissue. However, no studies appear to have been conducted on whether IPostC also has a protective effect on steatotic livers. This study sought to investigate the effects of IPostC on the maintenance of liver function in normal and steatotic livers during the acute phase of I/R injury. We present the following article in accordance with the ARRIVE reporting checklist (available at https://dx.doi.org/10.21037/atm-21-2275).
Methods
Animal model, anesthesia, and surgical procedure
Male Wistar rats, weighing 230–280 g, were used in this study. To induce steatotic livers, the test animals were fed a commercial high-fat choline-deficient diet (TP36002, Trophic Animal Feed High-tech Co. Ltd., China) for 6 weeks. The progression in steatosis was examined macroscopically and confirmed by a histological examination. The control animals were fed a standard laboratory diet (TP 0010M, Trophic Animal Feed High-tech Co. Ltd., China). All animals were kept in a temperature-controlled environment with 12 h light/dark cycles and with water and food ad libitum until use. The animal experiments were performed under a project license granted by institutional Animal Care and Use Committee of the Air Force Medical University, in compliance with institutional guidelines for the care and use of animals.
Ketamine (50 mg/kg) and chlorpromazine (50 mg/kg) were used to anesthetize normal and steatotic animals. Established segmental hepatic I/R models were used (22). After a midline laparotomy, the ligamentous adhesions of the liver to the diaphragm were severed, and the liver was exposed. Using an atraumatic microvascular clip, the hepatic artery and portal vein of the left and median liver lobes (approx. 70% of the liver) were occluded during the ischemic period of the study. Removed the clamp and began reperfusion. This method of partial hepatic ischemia avoided mesenteric venous congestion by allowing outflow of the splanchnic circulation through the right and caudate lobes. SHAM group was the control group, and the rats were anesthetized and laparotomized, and the hepatic hilum vessels were dissected without the induction of ischemia. Rats in the I/R group received 30 min of clamping followed by 2 or 6 hours of reperfusion in left hepatic artery and left portal vein. IPostC + I/R group, received the same treatment as in the I/R group, but followed by 3 cycles of 30-s reperfusion and 30-s ischemia immediately after 30 min of ischemia. The animals were actively warmed with heat lamps to conserve body heat. Their abdomens were lightly packed with wet gauze to prevent fluid evaporation, and normal saline (1 mL) was administered intraperitoneally to compensate for intraoperative fluid loss before the incision was closed. At the end of the trial, the animals were killed by exsanguination. Blood samples were obtained from the inferior vena cava, centrifuged at 4,000 rpm for 3 min at 4 °C, and preserved at −80 °C for use. Liver samples were taken from the ischemic liver lobes and stored in 10% phosphate-buffered formalin for fixation or immediately frozen at −80 °C for future use.
Experimental design
To evaluate the effect of IPostC on steatotic livers subjected to hepatic I/R, the normal and obese (Ob) animals were randomly divided into the following three groups: (I) the control group (n=12) in which 6 normal and 6 Ob animals were anesthetized and laparotomized, and the hepatic hilum vessels were dissected without the induction of ischemia; (II) the I/R group (n=24) in which the animals were divided into one subgroup of 6 normal and 6 Ob animals that were operated on for 30 min of partial ischemia and then 2 h of reperfusion, and a second subgroup of 6 normal and 6 Ob animals that were operated on for 30 min of partial ischemia and then 6 h of perfusion; and (III) the IPostC plus I/R (IPostC + I/R) group (n=24) in which the animals were subdivided into 2 subgroups (n=12) of 6 normal and 6 Ob animals that received the same treatment as in the I/R group, but with 3 cycles of 30-s reperfusion and 30-s ischemia immediately after 30 min of ischemia.
To explore the mechanisms potentially involved in the protective effects of IPostC, serum samples and hepatic tissues (ischemic lobes) were harvested at predetermined times to evaluate lipid peroxidation, neutrophil infiltration, inflammatory factor releasing, and intracellular energy loss. For these purposes, malondialdehyde (MDA) was measured in liver tissues as an indicator of lipid peroxidation and myeloperoxidase (MPO) activity was measured as an indicator of neutrophil accumulation. TNF-α levels were evaluated in plasma samples, and the content of adenosine triphosphate (ATP) in liver tissues was also detected.
Biochemical determinations
Concentrations of liver marker enzymes
Hepatocellular injury was determined by measuring the serum levels of liver marker enzymes. Blood was taken in the presence of anticoagulants. Plasma concentrations of alanine transaminase (ALT) and aspartate transaminase (AST) were measured spectrophotometrically using an automated biochemical analyzer (Hitachi 7160, Hitachi Incorporated, Tokyo, Japan).
Inflammatory factor assay
Plasma TNF-α was measured using a specific rat TNF-α (R&D Systems, Minneapolis, MN) immunoassay. All assays used in this study have been verified in rats and were conducted in accordance with the manufacturer’s instructions.
Lipid peroxidation and antioxidant enzyme activity assay
Lipid peroxidation was used as an indirect method to measure oxidative damage induced by reactive oxygen species (ROS), and the content of lipid peroxidation in liver tissues was measured by the thiobarbiturate reaction to determine MDA formation (21). The concentrations of MDA, superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX) activities in liver tissues were determined using assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in accordance with each manufacturer’s protocol, respectively.
MPO assay
As a marker of polymorphonuclear neutrophil (PMN) infiltration, MPO activity in hepatic tissues was determined using the MPO assay kit (Nanjing Jiancheng Bioengineering Institute) in accordance with the manufacturer’s protocol. MPO activity was measured photometrically using 3,3',5,5'-tetramethylbenzidine as a substrate. Frozen liver tissues were macerated, homogenized, sonicated, and centrifuged at 4,000 g for 12 min at 4 °C. MPO activity in the supernatant was measured and calculated based on the change in absorbance at 460 nm. MPO activity was shown as units of MPO activity per gram of wet liver.
Measurement of hepatic tissue ATP
Immediately after 6 h of reperfusion, the liver samples were frozen in liquid nitrogen for ATP determination. ATP levels in ischemic liver tissues were assayed spectrophotometrically using an assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the manufacturer’s protocol.
Histology
The steatosis of each liver was assessed using red oil staining on frozen specimens following standard procedures. To estimate the extent of hepatic injury, hematoxylin and eosin stained sections were evaluated using an established point-counting method in a blinded fashion (22). The following grades were employed: grade 0: minimal or no proof of injury; grade 1: mild injury including cytoplasmic vacuolization and focal nuclear pyknosis; grade 2: moderate to severe injury with extensive nuclear pyknosis, hypereosinophilic cytoplasm, and loss of intercellular boundaries; and grade 3: severe necrosis, hepatic cord disintegration, hemorrhage, and neutrophil infiltration.
Statistical analysis
Data are presented as mean ± standard deviation. Differences were analyzed by conducting Student’s t-tests between two groups, and one-way analyses of variance (ANOVAs) or Fisher exact tests among three groups. A P value less than 0.05 was regarded as statistically significant
Results
Induction of steatotic livers
All of the animals fed the high-fat diet developed moderate (30% to 60%) steatotic livers. Conversely, none of the rats in the normal groups developed hepatic steatosis. There was no mortality in our model 6 weeks after the induction of steatosis. The animals maintained a normal body weight during the high-fat diet periods, and there was no significant difference between the groups. Liver histological examinations showed moderate macro-vesicular steatosis under light microscopy (see Figure 1).
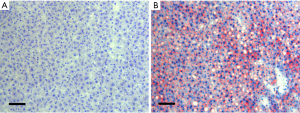
Hepatocellular injury
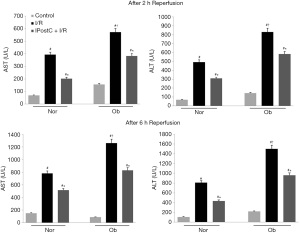
AST and ALT, which are established markers of hepatocellular injury, were measured in the normal and steatotic livers of rats after 2–6 h of reperfusion. I/R caused a significant rise in AST and ALT levels in the serum of both the normal and Ob rats. The AST and ALT levels were significantly higher in the Ob rats than the normal rats, indicating that I/R caused more severe liver damage in steatotic livers. IPostC of both steatotic and normal livers was effective at attenuating the damage caused by I/R, as evidenced by the decrease in serum AST and ALT levels (see Figure 2).
Inflammatory mediator and neutrophil infiltration
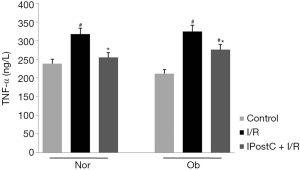
In relation to the beneficial effects of IPostC on hepatic I/R injury, the effects of TNF-α were also examined after 2 h of reperfusion. As Figure 3 shows, the increased TNF-α levels in I/R groups of both normal and Ob animals were reduced when IPostC was applied. However, no significant difference in TNF-α levels was observed between normal and Ob animals before or after reperfusion.
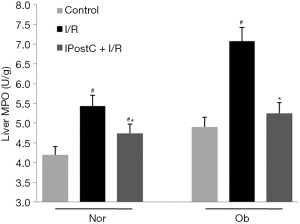
Neutrophil accumulation after liver reperfusion was determined by measuring MPO levels 6 h after liver reperfusion (see Figure 4). An increase in neutrophil accumulation was seen in both normal and steatotic livers. When IPostC was exerted after ischemia, hepatic MPO values were significantly lowered.
Lipid peroxidation and antioxidant enzyme activity
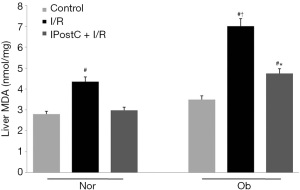
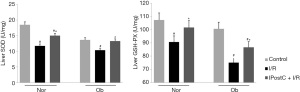
The effects of IPostC on lipid peroxidation and the antioxidant mechanism were evaluated after 6 h of reperfusion. As Figure 5 shows, high MDA levels were observed in I/R groups of normal and Ob animals. Further, the MDA levels in steatotic livers subjected to I/R were higher than those in normal livers. Conversely, MDA levels were reduced when IPostC was applied. As Figure 6 shows, the levels of SOD and GSH-PX activities in the I/R group were lower than those in the control group. However, when IPostC was applied, the levels of SOD and GSH-PX activities increased, and the SOD levels in the steatotic animals were similar to those in the control animals.
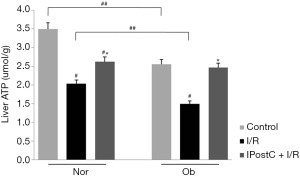
Hepatic tissue ATP
The effects of IPostC on tissue energy preservation were also investigated. ATP levels were measured in liver tissues after 6 h of reperfusion. As Figure 7 shows, compared with the control groups, I/R significantly decreased ATP levels in both normal and Ob animals, and ATP levels were significantly lower in steatotic livers than in normal livers before and after I/R injury. However, IPostC significantly preserved ATP levels, resulting in increased ATP levels in IPostC + I/R Group comparison to I/R group.
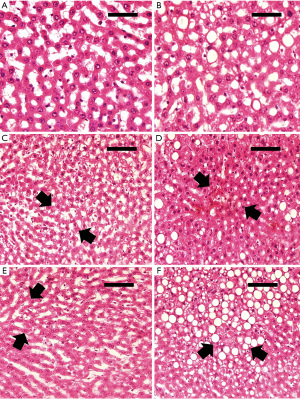
Histopathology
Parenchymal morphological changes were determined in liver sections stained with hematoxylin and eosin 6 h after reperfusion (see Figure 8). The results of the histological studies in the control group revealed no apparent lesions in either the normal or Ob animals (see Figure 8A,8B). However, at 6 h of reperfusion, the histological studies of normal livers undergoing I/R showed swelling and slight coagulative necrosis of hepatocytes with neutrophil infiltration, which were distributed over the entire hepatic parenchyma (see Figure 8C). Histological studies of steatotic livers showed severe multifocal areas of coagulative necrosis with neutrophil infiltration, randomly distributed in the liver parenchyma (see Figure 8D). In accordance with the biochemical test results, the range of necrosis and the number of necrotic areas were reduced in both normal and steatotic livers after performing IPostC (see Figure 8E,8F).
Discussion
Steatotic livers appear to have increased vulnerability to ischemic injury, and are considered a major risk factor for liver resection and transplantation. In previous studies, the biochemical and histological results indicated that compared to steatotic livers,normal livers tolerated more severe hepatic damage induced by hepatic ischemia (15,22-24). In addition, steatotic animals have shown decreased survival rates after hepatic I/R injury compared to normal animals (15). Due to the high prevalence of steatotic livers, it is essential to develop effective strategies to protect steatotic livers from hepatic I/R injury. The results of this study showed that IPostC, 3 cycles with 30 s of reperfusion and 30 s of ischemia conducted immediately after ischemia, provided protection against hepatic I/R injury upon 30 min of partial ischemia in normal and steatotic livers.
ROS production has been used as a key mechanism for I/R injury in many organs, including the liver (25). After warm ischemia, ROS were produced at the point of reperfusion, promoting leukocyte adhesion to the microvascular endothelium (22). Another important effect of uncontrolled ROS production is the peroxidation of cell membranes and other cellular lipids and proteins that can cause severe cellular injury (26). MDA is a product of free radical lipid peroxidation, and MDA levels may be used to measure the extent of lipid peroxidation in tissues (21). This study showed that lipid peroxidation increased in both normal and steatotic livers under 30 min of normothermic ischemia, and the increase was more prominent in steatotic livers. Thus, steatotic livers were found to be more susceptible to lipid peroxidation, which might be due to excessive fat or the generation of more ROS.
Our study also showed that IPostC inhibits the rise in MDA levels to a considerable extent; thus, IPostC has an antioxidant role in I/R. Given that the animals were randomly assigned to the groups, the livers of each Ob animal group should have shown a similar degree of hepatic steatosis. Thus, the effect of IPostC on lipid peroxidation can be interpreted in terms of reduced ROS production. SOD and GSH have multiple roles in cells, including in the maintenance of the oxidative antioxidant balance and the formation of conjugates with free radicals (27), and their activities indicate the body’s ability to scavenge free radicals. Our results showed that the activity of antioxidant enzymes decreased in both normal and steatotic livers after I/R injury, while the activity was significantly higher in the IPostC + I/R group. Thus, the IPostC-induced increase in antioxidant enzyme activity appears to contribute to the reduction of superoxide radicals after I/R injury in the liver.
TNF-α was shown to increase neutrophil aggregation, activate neutrophils to release inflammatory mediators, and lead to the overexpression of adhesion molecules on endothelial cells and leukocytes (28). Additionally, anti-TNF-α therapy could provide protection against hepatic I/R injury subjected to hepatic normothermic ischemia in steatotic livers (29). In the present study, increases of TNF-α were inhibited by IPostC in both normal and steatotic livers; however, no significant difference in the TNF-α levels was observed between the normal and Ob animals. Thus, in the present experimental model (30 min of 70% liver tissue ischemia), TNF-α did not appear to make steatotic livers more susceptible to hepatic I/R injury than normal livers. PMNs accumulated in the liver sinusoids may be essential effector cells in the pathogenesis of hepatic I/R injury, and they are the main source of ROS, which can cause cellular damage (10). The results of the present study showed I/R injury led to increases in MPO activity in both normal and steatotic livers; however, the increase was more prominent in steatotic livers. Additionally, our results suggest that IPostC could suppress an increase of MPO activity. Thus, the inhibition of TNF-α production may inhibit subsequent neutrophil activation. However, in IPreC studies, hepatic I/R injury was not found to be correlated with TNF-α production, and the advantage of IPreC could not be explained by a decrease in the release of TNF-α (15). In addition, microcirculatory disturbance appears to be one of the mechanisms by which steatotic livers are susceptible to I/R injury. Steatotic livers have reduced sinusoidal space due to fat infiltration, and inflammatory mediators, such as TNF-α and leukocytes, further reduce the sinusoidal space and impair microcirculation, resulting in reduced blood flow and prolonged ischemia (30).
The mechanisms of I/R injury in steatotic livers have not yet been thoroughly explored; a significant decrease in energy status may play a central role. ATP preservation during ischemia and ATP recovery during reperfusion may partially define hepatocyte viability and liver function (31). In addition, the existence of non-esterified fatty acids in steatotic livers is connected with a reduced capacity for ATP production (31). In the current study, the outcomes showed that ATP levels were lower in steatotic livers before or after reperfusion compared to normal livers. Thus, impaired hepatic energy metabolism already appears to be present in steatotic livers, but I/R injury is still not maintained. Our results demonstrated that IPostC significantly improved cellular bioenergetics in both normal and steatotic livers. Further, as ATP is essential for apoptosis, an increased susceptibility to I/R injury in the steatotic liver was related to the change from apoptosis, which is predominant in normal livers, to necrosis due to the depletion of intracellular ATP (32). In our study, necrosis was not a remarkable finding, as our protocol included a short ischemia period, and partial pedicle clamping to avoid intestinal blood flow stasis.
The underlying mechanisms by which IPostC could provide protective effects on the hepatic I/R injury in steatotic livers are largely unclear. We speculate that similar protective mechanisms to the hepatic I/R injury in normal livers could be also involved in steatotic livers, mainly including reduced oxygen free radicals, decreased neutrophil accumulation, increased antioxidant activity and enhanced mitochondrial function, during controlled reperfusion. In addition, several studies have also suggested that IPostC may increase nitric oxide (NO) concentrations and produce a cytoprotective environment, thereby reducing cell death and restoring hepatic function after reperfusion (28). Moreover, it has been shown that IPreC and IPostC had pronounced effects on gene expression during early reperfusion (33,34). IPreC and IPostC appear to mediate their protective roles by moderating the same genes and gene networks that are believed to be involved in sustaining cellular homeostasis (33).
In conclusion, IPostC could provide protection against normothermic I/R injury in normal and steatotic livers. ATP preservation and restoration may be one of the mechanisms of protection. These observations could have important clinical implications for the protection of normal and steatotic livers from hepatic I/R injury. Future studies should be conducted to examine the underlying biological mechanisms that may be involved in IPostC.
Acknowledgments
We would like to thank Ms. Regina Courtney for her help revising this manuscript.
Funding: This study is supported by the Health Research Fund Project of Shaanxi Province, China (No. 2018A014) and National Natural Science Foundation of China (NSFC) (No. 82072722).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-2275
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-2275
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-2275). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The animal experiments were performed under a project license granted by institutional Animal Care and Use Committee of the Air Force Medical University, in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol 2013;59:1094-106. [Crossref] [PubMed]
- Nastos C, Kalimeris K, Papoutsidakis N, et al. Global consequences of liver ischemia/reperfusion injury. Oxid Med Cell Longev 2014;2014:906965 [Crossref] [PubMed]
- Kato H, Kuriyama N, Duarte S, et al. MMP-9 deficiency shelters endothelial PECAM-1 expression and enhances regeneration of steatotic livers after ischemia and reperfusion injury. J Hepatol 2014;60:1032-9. [Crossref] [PubMed]
- Veteläinen R, van Vliet A, Gouma DJ, et al. Steatosis as a Risk Factor in Liver Surgery Ann Surg 2007;245:20-30. [Crossref] [PubMed]
- Nagai S, Fujimoto Y, Kamei H, et al. Mild hepatic macrovesicular steatosis may be a risk factor for hyperbilirubinaemia in living liver donors following right hepatectomy. Br J Surg 2009;96:437-44. [Crossref] [PubMed]
- McCormack L, Dutkowski P, El-Badry AM, et al. Liver transplantation using fatty livers: always feasible? J Hepatol 2011;54:1055-62. [Crossref] [PubMed]
- Nativ NI, Maguire TJ, Yarmush G, et al. Liver Defatting: An Alternative Approach to Enable Steatotic Liver Transplantation: Defatting Fatty Livers for Transplantation. Am J Transplant 2012;12:3176-83. [Crossref] [PubMed]
- Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology 2005;42:44-52. [Crossref] [PubMed]
- Llacuna L, Fernández A, Montfort CV, et al. Targeting cholesterol at different levels in the mevalonate pathway protects fatty liver against ischemia-reperfusion injury. J Hepatol 2011;54:1002-10. [Crossref] [PubMed]
- Ajamieh H, Farrell GC, McCuskey RS, et al. Acute atorvastatin is hepatoprotective against ischaemia-reperfusion injury in mice by modulating eNOS and microparticle formation. Liver Int 2015;35:2174-86. [Crossref] [PubMed]
- Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol 2016;13:193-209. [Crossref] [PubMed]
- Zarbock A, Schmidt C, Van Aken H, et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015;313:2133-41. [Crossref] [PubMed]
- Liu A, Fang H, Wei W, et al. Ischemic preconditioning protects against liver ischemia/reperfusion injury via heme oxygenase-1-mediated autophagy. Crit Care Med 2014;42:e762-71. [Crossref] [PubMed]
- Oberkofler CE, Limani P, Jang JH, et al. Systemic protection through remote ischemic preconditioning is spread by platelet-dependent signaling in mice. Hepatology 2014;60:1409-17. [Crossref] [PubMed]
- Serafín A, Roselló-Catafau J, Prats N, et al. Ischemic preconditioning affects interleukin release in fatty livers of rats undergoing ischemia/reperfusion. Hepatology 2004;39:688-98. [Crossref] [PubMed]
- Jiménez-Castro MB, Meroño N, Mendes-Braz M, et al. The effect of brain death in rat steatotic and non-steatotic liver transplantation with previous ischemic preconditioning. J Hepatol 2015;62:83-91. [Crossref] [PubMed]
- Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 2003;285:H579-88. [Crossref] [PubMed]
- Zhao H, Wang R, Tao Z, et al. Ischemic postconditioning relieves cerebral ischemia and reperfusion injury through activating T-LAK cell-originated protein kinase/protein kinase B pathway in rats. Stroke 2014;45:2417-24. [Crossref] [PubMed]
- Serviddio G, Romano AD, Gesualdo L, et al. Postconditioning is an effective strategy to reduce renal ischaemia/reperfusion injury. Nephrol Dial Transplant 2008;23:1504-12. [Crossref] [PubMed]
- Song X, Zhang N, Xu H, et al. Combined preconditioning and postconditioning provides synergistic protection against liver ischemic reperfusion injury. Int J Biol Sci 2012;8:707-18. [Crossref] [PubMed]
- Wang N, Lu JG, He XL, et al. Effects of ischemic postconditioning on reperfusion injury in rat liver grafts after orthotopic liver transplantation. Hepatol Res 2009;39:382-90. [Crossref] [PubMed]
- Serafín A, Roselló-Catafau J, Prats N, et al. Ischemic preconditioning increases the tolerance of Fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol 2002;161:587-601. [Crossref] [PubMed]
- Degli Esposti D, Sebagh M, Pham P, et al. Ischemic preconditioning induces autophagy and limits necrosis in human recipients of fatty liver grafts, decreasing the incidence of rejection episodes. Cell Death Dis 2011;2:e111 [Crossref] [PubMed]
- Rolo AP, Teodoro JS, Peralta C, et al. Prevention of I/R injury in fatty livers by ischemic preconditioning is associated with increased mitochondrial tolerance: the key role of ATPsynthase and mitochondrial permeability transition. Transpl Int 2009;22:1081-90. [Crossref] [PubMed]
- Jaeschke H, Woolbright BL. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant Rev (Orlando) 2012;26:103-14. [Crossref] [PubMed]
- Steenks M, van Baal MCPM, Nieuwenhuijs VB, et al. Intermittent ischaemia maintains function after ischaemia reperfusion in steatotic livers. HPB 2010;12:250-61. [Crossref] [PubMed]
- Agarwal A, Aponte-Mellado A, Premkumar BJ, et al. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 2012;10:49. [Crossref] [PubMed]
- Guo JY, Yang T, Sun XG, et al. Ischemic postconditioning attenuates liver warm ischemia-reperfusion injury through Akt-eNOS-NO-HIF pathway. J Biomed Sci 2011;18:79. [Crossref] [PubMed]
- Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis 2001;21:105-13. [Crossref] [PubMed]
- Hafez TS, Glantzounis GK, Fusai G, et al. Intracellular oxygenation and cytochrome oxidase C activity in ischemic preconditioning of steatotic rabbit liver. Am J Surg 2010;200:507-18. [Crossref] [PubMed]
- Selzner N, Selzner M, Jochum W, et al. Ischemic preconditioning protects the steatotic mouse liver against reperfusion injury: an ATP dependent mechanism. J Hepatol 2003;39:55-61. [Crossref] [PubMed]
- Esposti DD, Domart MC, Sebagh M, et al. Autophagy is induced by ischemic preconditioning in human livers formerly treated by chemotherapy to limit necrosis. Autophagy 2010;6:172-4. [Crossref] [PubMed]
- Knudsen AR, Kannerup AS, Dich R, et al. Ischemic pre- and postconditioning has pronounced effects on gene expression profiles in the rat liver after ischemia/reperfusion. Am J Physiol Gastrointest Liver Physiol 2012;303:G482-9. [Crossref] [PubMed]
- Knudsen AR, Kannerup AS, Grønbæk H, et al. Effects of ischemic pre- and postconditioning on HIF-1α, VEGF and TGF-β expression after warm ischemia and reperfusion in the rat liver. Comp Hepatol 2011;10:3. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


