
This article has an erratum available at: http://dx.doi.org/10.21037/atm-2023-17 the article has been update on 2023-07-20 at here.
Associations between the exposure to organophosphate flame retardants during early pregnancy and the risk of spontaneous abortion based on metabolomics combined with tandem mass spectrometry
Introduction
Spontaneous abortion (SAB), a spontaneous loss of pregnancy prior to 20 weeks of gestation, is one of the most common complications in pregnancy. Although some women may be able to achieve pregnancy, they may not be able to maintain that pregnancy throughout gestation and deliver a live-born infant. SAB has an occurrence rate of approximately 10%, while that of recurrent SAB is approximately 5% (1). There are many factors that affect early pregnancy abortion. Numerous investigations have indicated that chromosomal abnormalities, antiphospholipid syndrome, congenital structural abnormalities of the uterus, type I diabetes, and thyroid dysfunction may lead to spontaneous abortion in early pregnancy (2). However, other than these known risk factors, still almost half of the cause spontaneous abortion are unclear and further explanation is urgently needed. While the underlying causes of many adverse birth outcomes remain unclear, there is growing evidence that environmental factors can play an important role, which may include smoking, alcohol use, nutrition, air pollution, etc. (3). Developing fetuses are considered to be particularly vulnerable to environmental pollution. Several studies have shown that exposure to ambient air pollution (example particulate matter: PM2.5) during pregnancy is associated with low birth weight, preterm delivery, and other adverse health effects (4).
In recent years, Organophosphate flame retardants (OPFRs) have been increasingly used as alternative to polybrominated diphenyl ethers (PBDEs), which were formerly the most commonly used flame retardants until the mid-2000s. As a new class of flame retardant, OPFRs have been widely utilized in furniture, building materials, electronics, and other chemical processing materials. Since OPFRs are not covalently bound to materials used in products, they can slowly leach out into the environment during product usage. OPFRs are frequently detected in indoor air and dust, indicating that inhalation and ingestion of indoor dust is one of the major sources of human exposure. Moreover, the intake of food and drinking water from diet or skin direct contact with OPFR-containing products are the ways for the general population to absorb OPFRs (5-7). Some researchers found that OPFRs were identified as a new type of pollutant, as they were detected in environmental and biological samples. Long-term or short-term exposure to OPFRs may have adverse effects on human body, including: reproductive functions, neurodevelopmental outcomes, respiratory outcomes and immunotoxicity, etc. (8). Studies have shown that one or more OPFRs were detected in 90% of adult urine samples (9,10). Although exposure to OPFRs is ubiquitous, there is limited data about its potential impact on human pregnancy or fertility. The concentration of urinary OPFR metabolites could be used to assess human exposure. Carmen et al. monitored the pregnancy of women pregnant with assisted reproductive technology and found that an association between cycle specific urinary concentrations of the sum of OPFR metabolites and biochemical pregnancy loss. It is suggested that these potential associations may involve the early stage of implantation, decidualization, placental formation or embryogenesis, and may be mediated by uterus embryo hormone signal transduction (11). Although the research on their adverse effects on human health is limited, many studies have suggested that OPFRs and plasticizers may affect reproductive function and children’s health and development, such as neurodevelopment, asthma, obesity, etc. (12). In view of the fact that developing fetuses are particularly susceptible to a variety of toxicants, a high concentration of OPFRs and their metabolites in pregnant women has attracted increasing attention (13,14).
Metabolomics is based on qualitative and quantitative analyses of the end products in specific organisms or cells. The advantage of metabolomics is that metabolites are generated by ongoing biological processes in vivo, and thus reflect the phenotypic changes more accurately and directly. Metabolomics is widely used to identify potential biomarkers to help recognize and prevent diseases (15,16). It is gaining popularity as a tool to identify metabolic biomarkers, investigate pregnancy complications, and better understand the underlying mechanisms of disease at the metabolic level. Metabolomics studies the role of small-molecule metabolites in pregnancy and pregnancy-related diseases from a holistic perspective. It has been shown that different OPFRs cause the cellular metabolism disorder in different ways, including oxidative stress and the equilibrium of osmotic pressure, also caused mitochondrial dysfunction (17). In addition, cells exposed to OPFRs may also induce apoptosis through autophagy. In recent years, metabolomics has been increasingly applied to the study of gestational diseases (18,19). Urinary metabolomics plays a vital role in the study of gestational stages in human pregnancy. As one of the commonly used metabolomics biomaterials, urine is characterized by non-invasive collection methods and the presence of abundant metabolites, reflecting all of the biochemical pathways in the body.
Considering the differences in OPFR exposure levels between different countries (and even regions), it is critical to accurately measure the concentration of OPFRs in pregnant women in a given region and evaluate its effects on both the mother and fetus. In this study, we investigated the differences in urinary metabolic profiles between patients with SAB and women in normal early pregnancy who lived in Shanghai to identify and validate possible biomarkers of early SAB via Ultra high performance liquid chromatography-mass spectrometry (UHPLC-MS), and analyzed the potential relationship with OPFRs. Our results provide important new insights into the relationship between SAB and the environment from a metabolomics perspective.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/atm-21-3109).
Methods
Study design
From September 2019 to January 2020, a case-control study was conducted in the Shanghai First Maternity and Infant Hospital to study the effects of prenatal exposure to environmental pollutants on pregnant women. Participants were selected as follows: (I) those residing in the city of Shanghai; and (II) women who agreed to provide urine sample before 20 weeks of gestation. For each SAB case, one healthy control was randomly selected from among the pregnant woman with normal pregnancies in the hospital on the same day.
All participants provided demographic characteristics such as age, body mass index (BMI) before abortion, menstrual cycle regularity, the use of in vitro fertilization (IVF), parity, and history of abortion. BMI was calculated as weight (kilograms) divided by height (meters) squared.
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the Tongji University School of Medicine [Shanghai, China, No. SYXK (Shanghai) 2018-0038] and authorized by the Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine (Shanghai 201204, China) and informed consent was taken from all the patients.
Urine sample collection
Spot urine samples were collected from all participants by professional medical staff in the hospital. Midstream urine samples collected from the participants were aliquoted (1 mL ×4), frozen immediately, and stored at −80 °C until further processing.
Preparation for metabolomics analysis
Processing of urinary samples
The frozen samples were thawed on ice, and 200 µL of precooled methanol was added to 200 µL of each urine sample to extract metabolites. The extraction mixture was vortexed for 1 min, and then centrifuged at 11,000 rpm for 20 min at 4 °C. The supernatant was transferred to the Ultra high performance liquid chromatography -quadrupole time-of-flight mass spectrometry (UHPLC-Q-TOF/MS) analysis. 20 µL of each extraction mixture was mixed and treated according to the above process as a quality control (QC) sample.
UHPLC-Q-TOF/MS analysis conditions
Samples were analyzed using the Agilent 1290 Infinity liquid chromatography system equipped with Agilent 6538 accurate Mass quadrupole time-of-flight mass spectrometer (Agilent, USA). A poroshell 120 EC-C18 column (3.0 mm × 100 mm, 1.8 µm; Agilent, USA) was used for metabolite separation at 40 °C. The mobile phase was 0.1% formic acid–water (A) and 0.1% formic acid acetonitrile (B). The gradient elution was as follows: 10–30% B at 0–5 min, 30–80% B at 5–10 min, 80–95% B at 10–13 min, and 95% B at 13–15 min. The flow rate was 0.5 mL/min and the injection volume was 4 µL. Mass spectrometry was operated on Electrospray ion (ESI) source in both positive and negative ion modes using the following operating parameters: gas temperature, 350 °C; drying gas flow, 11 L/min; nebulizer pressure, 45 psi; fragmentor voltage, 120 V; skimmer voltage, 60 V; and capillary voltage, 4.0 kV (ESI+)/3.5 kV (ESI−). L-2-Chlorophenylalanine (2 µg/mL) was used as an internal standard (IS) to ensure mass accuracy and reproducibility. The full-scan data was acquired from m/z 50 to 1,100. Triple quadrupole mass spectrometry (MS/MS) analysis applied collision energy of 20 eV to study the structure of metabolites. QC samples were used to evaluate the stability of UHPLC-Q-TOF/MS during the entire acquisition period, and were analyzed six times at the beginning of the batch and then inserted among the real sample queue every nine samples.
Metabolomics data processing
Before the group data analysis, the acquired LC-MS raw data were converted to mzData formats with Agilent MassHunter Qualitative software (Agilent Technologies, USA) and analyzed using XCMS software (Scripps Research Institute, USA) implemented with R language, including retention time correction, peak identification, alignment, and annotation. The intensity of each peak was recorded, and a three-dimensional matrix containing arbitrarily assigned peak indices, sample names, and ion intensity information was generated. Features that were detected in <50% of the QC samples or 80% of the biological samples were removed.
The data were imported into SIMCA (V14.1; Umetrics, Sweden) for multivariate analysis after being log-transformed and normalized. Metabolite identification was based on the comparison of chromatographic characteristics (retention time) and spectral information (m/z, MS/MS fragments) obtained from the Human Metabolome Database (HMDB) database (http://www.hmdb.ca/) and Metabolite and Tandem MS Database (METLIN) database (http://metlin.scripps.edu/). MetaboAnalyst (https://www.m and etaboanalyst.ca/) Kyoto Encyclopedia of Genes and Genomes (KEGG) database (https://www.kegg.jp/) was used to analyze the pathway.
Preparation for measurement of OPFR metabolites
Processing of urinary samples
The collected samples were thawed at the room temperature, and OPFR metabolites were extracted from 400 µL of each sample using 140 µL of acetate buffer (1 mol/L, pH=5) and 40 µL of β-glucuronidase solution, spiked with 20 µL of tolbutamide (TBTM) solution (IS, 100 ng/mL). The mixture was extracted using 900 µL of acetonitrile, vortexed for 1 min, and subsequently incubated on a shaker at 37 °C overnight. Next, the mixture underwent centrifugation at 13,000 rpm for 10 min, and 1400 µL of supernatant was separated and subsequently concentrated by rotary distillation. The residue was re-dissolved in 100 µL of 50% methanol, then centrifuged at 11,000 rpm for 3 min, and the supernatant was transferred for LC-MS analysis.
UHPLC-MS/MS analysis conditions
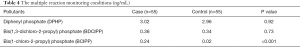
Urinary concentrations of OPFRs including diphenyl phosphate (DPHP), bis (1,3-dichloro-2-propyl) phosphate (BDCIPP) and bis (1-chloro-2-propyl) phosphate (BCIPP) were analyzed using the Agilent 1290 Infinity LC system equipped with Agilent 6470 Triple quadrupole mass spectrometer (Agilent, USA). XBridge C18 column (3.0 ×100 mm, 3.5 µm; Waters, USA) was used for chromatographic separation at 25 °C with the mobile phase of water containing 10 mM ammonium acetate (A) – acetonitrile (B). The gradient elution was as follows: 20–90% B at 0–3 min, and 90% B at 3–5 min. The flow rate was 0.5 mL/min and the injection volume was 2 µL. Mass spectrometry was operated on ESI source, with a negative ion mode at a drying gas flowing rate of 11 L/min and atomizing gas pressure of 40 psig. The multiple reaction monitoring conditions of the three urinary OPFRs are shown in Table 1.

Full table
Statistical analysis
Comparing the demographic characteristics of the participants, the Chi-square test was used for categorical variables between SAB cases and controls, and the Student’s t-test was used for continuous variables. For the concentration of urinary OPFR metabolites below the limits of detection (LOD), a value equal to one-half of the LOD was assigned for statistical analysis. The Student’s t-test was performed to compare differences between the two groups. Difference in the urine metabolome between the SAB and control groups were explored using orthogonal partial least squares discrimination analysis (OPLS-DA) conducted by SIMCA. The relative concentration of these metabolites was evaluated by the peak intensity of corresponding metabolites, which was normalized by the internal standard. The difference between the SAB case and control groups were examined using the Student’s t-test, and several criteria were defined to identify potential candidate biomarkers from our datasets: P<0.05 and variable influence in projection (VIP) >2.
Results
Demographic characteristics of study subjects
In total, 55 SAB cases and 55 healthy controls were enrolled during the study period. Cases that did not meet the conditions of the participants which formulated in the “experimental design” were excluded. The participants’ detailed characters are shown in Table 2. There were no significant differences between the case and control groups in terms of maternal age, gestational age, BMI, parity, and history of abortion.
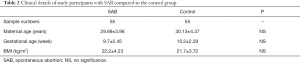
Full table
Selection of potential biomarkers and metabolic pathways analysis
In this study, to explore the metabolic changes in urine between women with first trimester SAB and those admitted for delivery beyond 24 weeks of gestation, an untargeted metabolomics analysis was performed. The total ion chromatograms (TIC) of positive-ion mode and negative-ion mode were analyzed using UHPLC-Q-TOF/MS, and the chromatographic separation spectra of QC samples exhibited good overlapping. The TICs presented an overview of the metabolic changes in urine between the first trimester SAB group and the control group based on positive and negative-ion analysis. The results showed that there were significant differences in urinary metabolites between the SAB and control groups.
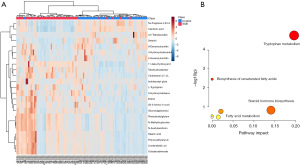
As shown in the UHPLC-Q-TOF/MS characteristic chromatograms, 1,313 ion peaks from the positive-ion mode and 3,915 ion peaks from the negative-ion mode were obtained via calibrating and integrating mass ions using XCMS software. Principal component analysis (PCA) was used to determine the sample separation and aggregation between the SAB and control groups. The results demonstrated that urine from SAB and normal gestation subjects had different metabolic characteristics. Supervised OPLS-DA was conducted to identify variables that differed between the SAB and normal gestation groups. The OPLS-DA score plot demonstrated a clearer separation of the urine between SAB and normal gestation samples (Figure 1). The statistically significant metabolites contributing to the model were chosen via the VIP (a threshold value of 2.0 was used in our study).

To precisely select potential biomarkers for further study, P values less than 0.05 were considered to be significant. Finally, 25 differential metabolites were annotated using exact mass data (m/z) from the KEGG and HMDB databases. Eight differential metabolites were found in the positive-ion mode, while 17 were found in the negative-ion mode (Table 3). The related metabolic pathway analysis and heatmap was performed on MetaboAnalyst (https://www.metaboanalyst.ca/). In the present study, abnormal changes were detected in tryptophan metabolism, lipid metabolism, steroid hormone biosynthesis, and fatty acid metabolism (Figure 2).
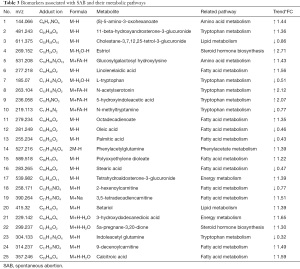
Full table
Urinary OPFR metabolite concentrations
The results of the 110 urine samples from this biomonitoring study in the Shanghai First Maternity and Infant Hospital are shown in Table 4. BCIPP was detected below the limit of detection (LOD, 0.01 ng/mL) in 32 of 55 urine samples from the control group, whereas all of the urine samples from the miscarriage case group contained BCIPP concentrations above the limit of quantitation (LOQ, 0.02 ng/mL). In the miscarriage case group, the average urine concentration was 0.24 ng/mL and the maximum concentration was 1.67 ng/mL. Meanwhile, in the control group, the average concentration was only 0.02 ng/mL, and this difference between the two groups was statistically significant.
Full table
Association between urinary SAB metabolites with OPFRs
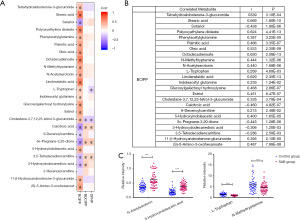
To contextualize the biological relevance of different metabolomes between SAB patients and women in normal early pregnancy, we integrated the individual metabolites into biochemical pathways for pathway enrichment analysis, and revealed the degree of change within each metabolite. We identified changes mostly in tryptophan and fatty acid pathways associated with diverse physiological processes. We correlated the levels of individual metabolites to the OPFR metabolite concentrations (Figure 3), and the result provided suggestive evidence of an association between urinary concentrations of OPFRs and endogenous metabolites.
Discussion
The etiology of SAB is complex, and includes chromosome genetic alterations, immune dysfunction, infections, male factors, uterine abnormalities, and environmental factors. In our previous study, we found that the concentrations of OPFRs and their metabolites were different between SAB case and control groups. We also observed that higher urinary BDCIPP levels were related to increased risk of fetal chromosome abnormalities. Although there is no studies have examined the impact of prenatal exposure to OPFRs on fetal chromosome, some previous studies conducted in zebrafish have revealed that exposure to TDCIPP (parent compound of BDCIPP) at different stages of embryonic development could induce a variety of abnormal phenotypes (20). In this study, we are interested in whether these differences are related to the metabolism of SAB patients. Consequently, in this study, an Ultra high performance liquid chromatography-Quadrupole-Time of flight mass spectrometry (UHPLC-Q-TOF/MS)-based metabolomic approach and Ultra high performance liquid chromatography—Triple quadrupole mass spectrometry (UHPLC-MS/MS)-based OPFR metabolites determination were employed to investigate the potential interaction between OPFRs and urine metabolites in a cohort of 110 pregnant women during early pregnancy. Using the UHPLC-Q-TOF/MS-based urine metabolomics approach, 25 potential biomarkers related to SAB were identified by positive and negative ion patterns. Metabolic pathway analysis helps us to understand the underlying molecular functions of these biomarkers in urine metabolism. These potential metabolites were found to be primarily involved in tryptophan metabolism, unsaturated fatty acids biosynthesis, steroid hormone biosynthesis, and other pathways. Using the UHPLC-MS/MS with a negative ion multiple reaction monitoring (MRM) mode, the vast majority of pregnant women included in our study population had detectable levels of several OPFRs in their urine samples. Statistical analysis showed that there was no significant difference between the levels of DPHP and BDCIPP in the SAB and control groups. However, the BCIPP concentration in the SAB group was significantly higher than that in the control group, with a mean value of 0.24 and 0.02 ng/mL, respectively, which was significantly different. A previous study reported that the concentrations of urinary OPFRs was associated with reduced probability of fertilization, implantation, clinical pregnancy, and live birth (21). Similarly, our results also indicated a correlation between SAB and OPFRs. Correlation analysis results showed that among three OPFRs, BCIPP exhibited moderate correlations with most metabolites associated with SAB.
Tryptophan (Trp), as a precursor of a variety of bioactive metabolites, plays an important role in the placenta, fetal development, and immune regulation during pregnancy. Previous studies have confirmed that tryptophan metabolism changes significantly in the maternal body during normal pregnancy (22). In our study, there was a significant change in tryptophan metabolism between SAB and normal pregnancy, which indicated fluctuations of neurotransmitters due to metabolic adaption in the maternal body for the growth of the fetus. Previous studies revealed that the expression and activity of indoleamine 2,3-dioxygenase (IDO) in patients with unexplained recurrent SAB was low, which suggested that IDO may be closely related to recurrent spontaneous abortion (RSA) (23). Previous studies have also shown that intake of serotonin reuptake inhibitors (SSRIs) in early pregnancy can reduce the pregnancy rate of women and has a statistically significant association with low birth weight (24). In our study, the level of 5-hydroxyindoleacetic acid in the urine of the SAB group was significantly higher than that of control group, and there was a moderate correlation between the level of 5-hydroxyindoleacetic acid and the concentration of BCIPP. Therefore, we speculate that this correlation may be related to the metabolism of 5-hydroxytryptamine in vivo. In addition, N-Acetylserotonin and N-methyltryptamine are intermediates involved in tryptophan metabolism. BCIPP exhibited significant correlations with N-Acetylserotonin and N-methyltryptamine, which indicated that OPFRs may also affect tryptophan metabolism through interactions with key enzymes, such as IDO, methyltransferase, and arylalkylamine N-acetyltransferase in the metabolic pathways of N-Acetylserotonin and N-methyltryptamine in the maternal body.
Fatty acids are essential nutrients for normal embryo development and play an important role in oocyte maturation and embryonic development. Fatty acids are classified as saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) depending on their molecular structure (25). In this study, the levels of oleic acid, palmitic acid, and stearic acid in the SAB group was significantly lower than those in control group, and there was a statistically significant correlation between the level of fatty acids and urinary BCIPP. Previous studies have suggested a positive association between total MUFAs and pregnancy/live birth, and an inverse association with loss of pregnancy (26). Oleic acid has been identified as a blastomeric and postcryo preservation survival biomarker in bovine samples, indicating that MUFAs may have a positive effect on embryo development. Current knowledge suggests that the potential mechanisms of oleic acid with female reproduction may include altered metabolic channeling, plasma membrane fluidity, and oxidative stress. In addition, oleic acid and palmitic acid are raw materials for linoleic acid synthesis. Linoleic acid is an essential fatty acid, which is preferentially metabolized into arachidonic acid and thromboxane (27,28). The concentrations of oleic acid, saturated fatty acid, palmitic acid, and stearic acid in the urine of SAB patient’s decreases, which may be related to maintaining linoleic acid balance in the body. In our study, the urinary BCIPP concentration was significantly correlated with the levels of various fatty acids. Therefore, it is reasonable to speculate that the effect of OPFRs on early pregnancy may be related to their interference with fatty acid synthesis and metabolism.
Concluding remarks
In this study, we employed an integrated approach of UHPLC-Q-TOF/MS-based metabolomics and UHPLC-MS/MS-based OPFRs determination to obtain urine metabolites between early SAB and healthy pregnant women, and then evaluated the association between OPFRs and these metabolites. As potential biomarkers, these metabolites may have great value in evaluating OPFR exposure in healthy pregnant women. The results of correlation analysis showed that metabolites in the metabolic networks of tryptophan and fatty acid metabolism were correlated with the levels of urinary OPFRs during early pregnancy. Among them, tryptophan metabolism, as a potential marker pathway of SAB, was presented in this study, and its intermediate metabolites were significantly related to BCIPP level, indicating that the effect of OPFRs on maternal metabolism in early pregnancy may be related to tryptophan metabolism. The correlations between OPFRs and endogenous metabolites provide important baseline data on the mechanism of OPFRs action in the maternal body of healthy pregnant women during the course of normal pregnancy.
However, in the process of demographic characteristics registration, the comprehensive information of participants, including working environment, working intensity, eating habits, mental state, and other information could not be fully understood, and the influence of these factors cannot be completely excluded. Therefore, we can only infer that the different metabolites may be related to the interference of OPFRs. The direct effect of flame retardants on these metabolites requires further study. In addition, there is no relevant report on the difference of serum metabolomics between spontaneous abortion women and healthy women exposed to OPFRs in early pregnancy. Our research group is trying to collect serum samples and the related research of serum metabolomics is our next work.
Acknowledgments
Funding: This work was financially supported by the National Natural Science Foundation of China (81803184), the Shanghai Sailing Program (18YF1419600).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-3109
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-3109
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-3109). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the Tongji University School of Medicine [Shanghai, China, No. SYXK (Shanghai) 2018-0038] and authorized by the Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine (Shanghai 201204, China) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ng KYB, Cherian G, Kermack AJ, et al. Systematic review and meta-analysis of female lifestyle factors and risk of recurrent pregnancy loss. Sci Rep 2021;11:7081. [Crossref] [PubMed]
- Bahia W, Soltani I, Abidi A, et al. Identification of genes and miRNA associated with idiopathic recurrent pregnancy loss: an exploratory data mining study. BMC Med Genomics 2020;13:75. [Crossref] [PubMed]
- Vardavas CI, Chatzi L, Patelarou E, et al. Smoking and smoking cessation during early pregnancy and its effect on adverse pregnancy outcomes and fetal growth. Eur J Pediatr 2010;169:741-8. [Crossref] [PubMed]
- Grippo A, Zhang J, Chu L, et al. Air pollution exposure during pregnancy and spontaneous abortion and stillbirth. Rev Environ Health 2018;33:247-64. [Crossref] [PubMed]
- Hoffman K, Lorenzo A, Butt CM, et al. Predictors of urinary flame retardant concentration among pregnant women. Environ Int 2017;98:96-101. [Crossref] [PubMed]
- Wei GL, Li DQ, Zhuo MN, et al. Organophosphorus flame retardants and plasticizers: sources, occurrence, toxicity and human exposure. Environ Pollut 2015;196:29-46. [Crossref] [PubMed]
- Hoffman K, Garantziotis S, Birnbaum LS, et al. Monitoring indoor exposure to organophosphate flame retardants: hand wipes and house dust. Environ Health Perspect 2015;123:160-5. [Crossref] [PubMed]
- Wang C, Chen H, Li H, et al. Review of emerging contaminant tris(1,3-dichloro-2-propyl)phosphate: Environmental occurrence, exposure, and risks to organisms and human health. Environ Int 2020;143:105946 [Crossref] [PubMed]
- Butt CM, Congleton J, Hoffman K, et al. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ Sci Technol 2014;48:10432-8. [Crossref] [PubMed]
- Hoffman K, Butt CM, Webster TF, et al. Temporal Trends in Exposure to Organophosphate Flame Retardants in the United States. Environ Sci Technol Lett 2017;4:112-8. [Crossref] [PubMed]
- Messerlian C, Williams PL, Mínguez-Alarcón L, et al. Organophosphate flame-retardant metabolite concentrations and pregnancy loss among women conceiving with assisted reproductive technology. Fertil Steril 2018;110:1137-1144.e1. [Crossref] [PubMed]
- Peng B, Yu ZM, Wu CC, et al. Polybrominated diphenyl ethers and organophosphate esters flame retardants in play mats from China and the exposure risks for children. Environ Int 2020;135:105348 [Crossref] [PubMed]
- Luo D, Liu W, Tao Y, et al. Prenatal Exposure to Organophosphate Flame Retardants and the Risk of Low Birth Weight: A Nested Case-Control Study in China. Environ Sci Technol 2020;54:3375-85. [Crossref] [PubMed]
- Rock KD, St Armour G, Horman B, et al. Effects of Prenatal Exposure to a Mixture of Organophosphate Flame Retardants on Placental Gene Expression and Serotonergic Innervation in the Fetal Rat Brain. Toxicol Sci 2020;176:203-23. [Crossref] [PubMed]
- Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol 2016;17:451-9. [Crossref] [PubMed]
- Wishart DS. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol Rev 2019;99:1819-75. [Crossref] [PubMed]
- Hao Z, Zhang Z, Lu D, et al. Organophosphorus Flame Retardants Impair Intracellular Lipid Metabolic Function in Human Hepatocellular Cells. Chem Res Toxicol 2019;32:1250-8. [Crossref] [PubMed]
- Bracewell-Milnes T, Saso S, Abdalla H, et al. Metabolomics as a tool to identify biomarkers to predict and improve outcomes in reproductive medicine: a systematic review. Hum Reprod Update 2017;23:723-36. [Crossref] [PubMed]
- Clinton CM, Bain JR, Muehlbauer MJ, et al. Non-targeted urinary metabolomics in pregnancy and associations with fetal growth restriction. Sci Rep 2020;10:5307. [Crossref] [PubMed]
- Dasgupta S, Cheng V, Vliet SMF, et al. Tris(1,3-dichloro-2-propyl) Phosphate Exposure During the Early-Blastula Stage Alters the Normal Trajectory of Zebrafish Embryogenesis. Environ Sci Technol 2018;52:10820-8. [Crossref] [PubMed]
- Carignan CC, Mínguez-Alarcón L, Butt CM, et al. Urinary Concentrations of Organophosphate Flame Retardant Metabolites and Pregnancy Outcomes among Women Undergoing in Vitro Fertilization. Environ Health Perspect 2017;125:087018 [Crossref] [PubMed]
- Karahoda R, Abad C, Horackova H, et al. Dynamics of Tryptophan Metabolic Pathways in Human Placenta and Placental-Derived Cells: Effect of Gestation Age and Trophoblast Differentiation. Front Cell Dev Biol 2020;8:574034 [Crossref] [PubMed]
- Fei H, Hou J, Wu Z, et al. Plasma metabolomic profile and potential biomarkers for missed abortion. Biomed Chromatogr 2016;30:1942-52. [Crossref] [PubMed]
- Mistry AM, Vidal G, Voogt JL. Dopaminergic and serotonergic activity in the hypothalamus during early and late pregnancy. Brain Res 1991;550:239-46. [Crossref] [PubMed]
- Li K, Zhang X, Chen G, et al. Association of fatty acids and lipids metabolism in placenta with early spontaneous pregnancy loss in Chinese women. Food Funct 2018;9:1179-86. [Crossref] [PubMed]
- Kim K, Browne RW, Nobles CJ, et al. Associations Between Preconception Plasma Fatty Acids and Pregnancy Outcomes. Epidemiology 2019;30:S37-46. [Crossref] [PubMed]
- Fayezi S, Leroy JLMR, Ghaffari Novin M, et al. Oleic acid in the modulation of oocyte and preimplantation embryo development. Zygote 2018;26:1-13. [Crossref] [PubMed]
- Song J, Wang X, Guo Y, et al. Novel high-coverage targeted metabolomics method (SWATHtoMRM) for exploring follicular fluid metabolome alterations in women with recurrent spontaneous abortion undergoing in vitro fertilization. Sci Rep 2019;9:10873. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


