
Cannabinoid receptor agonist WIN55212-2 reduces unpredictable mild stress-induced depressive behavior of rats
Introduction
Depression, also known as depressive disorder, is a common mental illness, mainly characterized by low mood and loss of interest and pleasure, resulting in increased fatigue, lessened activity, and reduced energy. It can be accompanied by varying degrees of cognitive and behavioral changes (1), all of which can be persistent or recurrent, severely impairing work, study, and the daily life ability of patients, even causing suicide when the situation worsens (2). Although treatments for depression have existed for decades, the remission rate is low, the recurrence rate is high, and the cure rate of the disease has not increased significantly. According to statistics, only 12.7% of patients worldwide have received minimal treatment (3). As of 2019, depression had affected approximately 300 million people worldwide and was the main affecting factor of the global disability rate. Its average mortality rate is 2–3 times that of the general population, which means that life expectancy is shortened by 10 to 20 years (4). Many depression patients also suffer from other complications that seriously affect their health, such as cardiovascular disease and obesity, among others (5), imposing a great economic burden to society. Therefore, the treatment and prevention of depression remain urgent public health problems (6).
In the past two decades, research regarding the pathogenesis of depression has focused on the inflammatory theory of depression, which posits that depression is a neuroimmune mental disorder mediated by cytokines (7,8). Clinical evidence shows that immune-related diseases such as cardiovascular diseases, autoimmune diseases, and multiple sclerosis are associated with a higher comorbidity rate with depression (9). A meta-analysis of inflammatory indicators also highlighted that the tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and C-reactive protein (CRP) levels in the peripheral serum and central nervous system (CNS) of patients with depression are significantly increased (10,11), but can be reduced by antidepressant treatment (12). The results of animal models show that the injection of lipopolysaccharide (LPS) and exogenous pro-inflammatory cytokines can induce depressive behavior such as anhedonia (13). Furthermore, inflammatory cytokines play a central role in the immune system and inflammatory response. In addition to coordinating the communication between cells, they can also change neurochemical and neuroendocrine processes, thereby exerting wide-ranging effects on cognition, mood, and behavior. All the above studies have indicated that neuroinflammation is closely related to the pathogenesis of depression, and inflammation of the CNS is one of the important pathophysiological mechanisms that contributes to the occurrence of depression (14).
The endocannabinoid signaling system plays an important role in the regulation of various neurological functions in the CNS, including learning and memory, pain perception, and the regulation of intracellular signals (15,16), as well as synaptic transmission and synaptic plasticity in the CNS (17). The endocannabinoid signaling system consists of four parts: cannabinoid receptors, endocannabinoids, enzymes that produce and hydrolyze endocannabinoids, and endocannabinoid transporters. Cannabinoid receptors are a class of seven-pass transmembrane G protein-coupled receptors which mainly include two different subtypes: type 1 cannabinoid receptor (CB1R) and type 2 cannabinoid receptor (CB2R) (18,19). CB1R is abundantly expressed in multiple areas of the CNS, while CB2R is mainly expressed in the immune system (20). Many studies have suggested that the use of CB1R agonists can reduce depressive behavior (21,22), while the mechanism by which CB1R agonists prevent and treat the disease is unclear. To this end, we used chronic unpredictable mild stress (CUMS) to establish a rat model of depression, and observed the effects of the CB1R agonist WIN55212-2 on rat behavior and the neuroinflammation caused by the NF-κB signaling pathways to ultimately explore the treatment effects and mechanisms of CB1R agonists on the depressive behavior of rats. We present the following article in accordance with the ARRIVE reporting checklist (available at https://dx.doi.org/10.21037/atm-21-3143).
Methods
Establishment of the CUMS-induced depressive rat model
The establishment of the CUMS depression model referred to a method in the literature (23). The details are as follows, including a total of 11 kinds of stress: (I) wet litter; (II) water-prohibited overnight; (III) fasting overnight; (IV) tilting the cage at 45° for 24 hours; (V) flashing lights at night; (VI) day/night light cycle reversal; (VII) overnight in a crowded cage; (VIII) odorous environment; (IX) 80 dB white noise lasting for 3 hours; (X) swimming in cold at 8 °C for 5 minutes; (XI) restricted behaviors for 4 hours. Two types of stressors were randomly used every day, and the same stress method was not allowed to be used continuously. The normal group was raised with 10 animals/cage and normally fed with water, and the other groups were raised with 1 animal/cage and received various stimulations: fasting for 1 day, water deprivation for 1 day, continuous stimulation for 28 days, and 1 type was randomly selected every day. Experiments were performed under a project license (No. C202106-8) granted by the Ethics Committee of Guangdong Medical Experimental Animal Center, in compliance with Chinese guidelines for the care and use of animals. A protocol was prepared before the study without registration.
Experimental grouping
Thirty-six male Sprague-Dawley (SD) rats aged 6–8 weeks weighing 180–220 g were randomly divided into six groups, with 6 rats in each group: (I) control group: after 1 week of adaptive culture, no pressure was applied to the rats, and all the rats were gavaged the same amount of saline once a day for 4 consecutive weeks; (II) CUMS group: after 1 week of adaptive culture, rats were treated at the same time as applying CUMS. All the rats were gavaged the same amount of saline once a day for 4 consecutive weeks; (III) fluoxetine (Flu) positive control group: rats were treated at the same time as applying CUMS, and fluoxetine at 10 mg/kg was injected intraperitoneally once a day for 4 consecutive weeks; (IV) CB1R agonist WIN55212-2 (WIN) group: rats were treated at the same time as applying CUMS, and WIN55212-2 at 0.5 mg/kg was injected intraperitoneally once a day for 4 consecutive weeks; (V) NF-κB inhibitor (PDTC) group: rats were treated at the same time as applying CUMS, and PDTC at 100 mg/kg was intraperitoneally injected once a day for 4 weeks; (VI) WIN + PDTC group: rats were treated at the same time as applying CUMS, and WIN55212-2 at 0.5 mg/kg and PDTC at 100 mg/kg were intraperitoneally injected once a day for 4 consecutive weeks. After 4 weeks of stimulation, the serum and brain hippocampal tissues of rats were obtained.
Sucrose preference test (SPT)
The SPT was used to detect anhedonia and evaluate depressive behavior in animals. The experiment was divided into two stages: the adaptation stage and the detection stage. For the adaptation stage, animals were given two bottles with the same appearance, both containing 1% sucrose water, and were allowed to drink and eat freely for 24 hours. Then, 1 bottle of sucrose water was replaced by a bottle with the same appearance containing clear water, and the rats were allowed to drink and eat freely for 24 hours. The animals were water-prohibited and fasted for 24 hours before testing. For the detection stage, animals were raised in single cages. Two identical bottles were randomly placed in each cage at the same time, containing either 1% sucrose water or clear water. The weight of each solution was recorded before placing it in the cage, and the position of the two bottles was changed after 12 hours. After 24 hours from the start of the test, bottles were removed, the weight of each solution was weighed, and the preference rate of sucrose water for each animal was calculated.
Behavioral experiments
Forced swim test (FST)
The FST is widely used in the study of antidepressant effects and mechanisms of depression. The rat was put into a limited space to swim. At the beginning, it tried hard to escape by swimming, but soon remained afloat, showing desperation. The time that the rat stayed afloat in the water was the immobility time. The accumulated immobility time of the last 4 minutes within the 6 minutes was recorded. The longer the accumulated immobility time, the more obvious the depressive behavior of the rat. The experimental device was a transparent plexiglass hollow cylinder (height 25 cm, diameter 14 cm) with water at 23–25 °C inside, and the water level was approximately 18 cm high.
Open field test (OFT)
The OFT is used to evaluate the spontaneous activities, exploratory behaviors, and spontaneous anxiety of rodents, also known as the open box experiment (24). A single rat was placed in the central area of the open box, and each rat was observed for 10 minutes. The experiment was carried out in a quiet room, with a camera at the top of the open box to observe and record all the activities. The behavioral software (the eth vision video tracking system) was used to track and analyze the behavioral video of the rats. Before testing, 75% ethanol was applied to clean the feces, inner walls, and the bottom of the open box to prevent any information left by the previous animal (such as the animal’s urine, feces, and smell) from affecting the test result. OFT recorded indicators included total distance, number of times entering the central area, and stay time in the central area.
Elevated plus maze (EPM) experiment
The primary mechanism of the EPM for evaluating the depressive behavior of animals involves utilizing the animal’s curiosity and fear (open arms) and dark preference (closed arms), forming a conflict between exploration and avoidance to investigate the depression and anxiety of animals (25). In the experiment, each rat was placed on the central platform of the plus maze with its head facing one of the closed arms. After releasing the rats, they were timed for 5 minutes and the following indicators were recorded: the number of times rats entered the open arm (OE), the number of times rats entered the closed arm (CE), the time rats stayed in the open arm (OT), and the time rats stayed in the closed arm (CT).
Enzyme-linked immunosorbent assay (ELISA)
Rat serum and hippocampal tissue were collected following the instructions of the IL-1β, IL-6, TNF-α, and cyclooxygenase-2 (COX-2) detection kits (R&D Systems). The absorbance value at a wavelength of 540 nm was recorded from the ELISA instrument, and the concentrations of IL-1β, IL-6, TNF-α, and COX-2 were calculated according to the standard curve.
Hematoxylin and eosin (HE) staining
Part of the hippocampal tissue from each group of rats after fixation was obtained and underwent the following steps: (I) dehydration, immersion, and embedding in wax; (II) sectioning into approximately 4 μm slices; (III) exhibition and baking; (IV) HE staining; (V) neutral gum mounting; (VI) imaging and analysis. The histopathological changes of the hippocampal tissues of rats in each group were confirmed and compared.
Nissl staining
Nissl staining is a method of staining nerve tissue with a basic dye, which mainly combines with nucleic acids. After euthanasia, the rats in each group were perfused to obtain tissues, during which squeezing the tissue was avoided to prevent the false appearance of neuron contraction. Frozen tissue sections were washed twice with distilled water for 30 seconds each time. Tissue sections were placed in a 0.5% toluidine blue staining tank, and remained in a 50–60 °C incubator for 30 minutes. Then, the sections were washed twice with distilled water, differentiated by 95% ethanol for 5 seconds, dehydrated with gradient alcohol, made transparent by xylene, and sealed with neutral gum.
Quantitative real-time PCR (qRT-PCR)
The qRT-PCR primers were designed according to the rat gene sequences published on NCBI: brain-derived neurotrophic factor (BDNF) F/R: 5'-CACCAGCCATGTAAACATCC-3', 5'-ATGCTTGTTCTCGTCTCTGT-3'; tyrosine kinase receptor B (TrkB) F/R: 5'-CCAGAGCCGTTGGTGTATC-3', 5'-TCAAGGCTTT-TCCATCCAAC-3'; inducible nitric oxide synthase (iNOS) F/R: 5'-GACAGCACCAC-CTACGAT-3', 5'-GGATCACTTCAATGGCCT-3'; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) F/R: 5'-CAAGCAACTGTCCCTGAG-3', 5'-TAG-ACAGAAGGTGGCACA-3'. All primers were synthesized by Shanghai Bioengineering Co., Ltd. The RNA from the hippocampal tissues of each group was extracted and reversely transcribed into cDNA, followed by the qPCR reaction. Reaction program: 95 °C for 3 min; 95 °C for 5 s, 60 °C for 30 s, 72 °C for 25 s, 35 cycles; 65 °C for 5 min; 95 °C for 50 s. GAPDH was selected as the internal reference gene. The 2–ΔΔCT method was used to calculate the relative expression of genes.
Western blot
Hippocampal tissue samples were collected, and protein lysis solution (RIPA) was used for lysing tissues on ice for 30 minutes to extract proteins. After protein quantification, 10% SDS-PAGE was performed, and the protein on the gel was transferred to a PVDF membrane by the wet transfer method. After blocking the PVDF membrane with blocking solution for 2 hours at room temperature, rabbit monoclonal antibody BDNF (1:1,000, ab108319), rabbit monoclonal antibody TrkB (1:1,500, ab18741), rabbit monoclonal antibody p-IKKβ (1:1,000, ab38515), rabbit monoclonal antibody IKKβ (1:1,000, ab124957), rabbit monoclonal antibody IκBα (1:1,000, ab32518), rabbit polyclonal antibody NF-κB p65 (1:1,500, ab16502), and rabbit polyclonal antibody GAPDH (1:1,000, ab9485, Abcam, Cambridge, UK) were applied and incubated overnight at 4 °C. The next day, membranes were washed with PBST for 30 minutes, then incubated with an HRP-labeled secondary antibody at 37 °C for 1 hour, and the membrane was washed with PBST for 1 hour. Finally, the target protein was detected using the enhanced chemiluminescence ECL kit, and the gray value was analyzed by Image J software.
Statistical analysis
All experiments were repeated at least 3 times. All data were expressed as mean ± standard deviation. All data were statistically analyzed by SPSS 18.0, and the t-test or one-way analysis of variance was performed. P<0.05 was considered to indicate a significant difference.
Results
WIN55212-2 reduced the weight loss of rats induced by CUMS
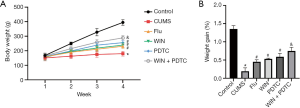
In order to explore whether WIN55212-2 had a corresponding effect on the weight of CUMS model rats, the rats were weighed every week, and the weight changes were calculated. The results showed that the body weight and weight changes of the rats in the CUMS group reduced significantly when compared with the control group. The body weight and weight changes of the rats in the Flu group, WIN group, and PDTC group increased significantly when compared with the CUMS group, while the body weight and weight changes of rats in the WIN + PDTC group increased significantly when compared with the WIN group (Figure 1A,B). The results suggested that the CB1R agonist WIN55212-2 and the inhibition of the NF-κB signaling pathway significantly reduced the weight loss of rats induced by CUMS.
WIN55212-2 reduced the CUMS-induced depressive behaviors of rats
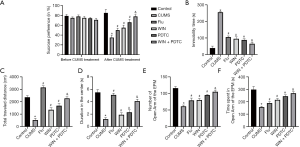
The results of the SPT showed that before CUMS, the sucrose preferences among rats of each group were not significantly different (P>0.05). After applying 4 weeks of CUMS to the rats, the sucrose preference of the rats from the CUMS group was significantly reduced when compared with the control group. The sucrose preference of the Flu group, WIN group, and PDTC group was significantly increased when compared with the CUMS group. The sucrose preference of the WIN + PDTC group was significantly increased when compared with the WIN group (Figure 2A). The behaviors of rats were tested, and the results of the FST showed that the immobility time of the CUMS group was significantly increased when compared with the control group (P<0.05). The immobility time of the Flu group, WIN group, and PDTC group was significantly shortened when compared with the CUMS group. The immobility time of the WIN + PDTC group was significantly shortened when compared with the WIN group (Figure 2B,C). The results of the OFT showed that the total movement distance and duration of the CUMS group were significantly reduced when compared with the control group (P<0.05). The total movement distance and duration of the Flu group, WIN group, and PDTC group were significantly increased when compared with the CUMS group. The total movement distance and duration of the WIN + PDTC group were significantly increased when compared with the WIN group (Figure 2D). The results of the EPM experiment showed that OE and the time they stayed in the open arm of the CUMS group were significantly reduced when compared with the control group. OE and the time they stayed in the open arm of the Flu group, WIN group, and PDTC group were significantly increased when compared with the CUMS group. OE and the time they stayed in the open arm of the WIN + PDTC group were significantly increased when compared with the WIN group (Figure 2E,F). All the above studies suggested that after treatment with WIN55212-2 or inhibition of the NF-κB signaling pathway, the CUMS-induced depressive behaviors of rats were reduced.
The protective effects of WIN55212-2 on hippocampal neuron damage caused by CUMS
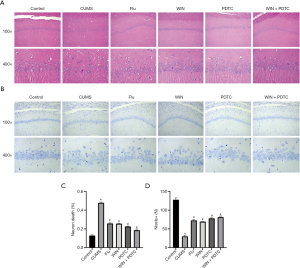
We subsequently analyzed the histopathology of the different groups. HE staining results showed that the hippocampal neurons of the control group had complete structures, regular cell morphology, normal intercellular spaces, neat arrangement and density, and clear nucleoli. Compared with the control group, the neurons of the CUMS group were atrophied and arranged loosely, the intercellular spaces were enlarged, some cells were vacuolated, and the nuclei were pyknotic. Compared with the CUMS group, the number of neurons in the hippocampus of the Flu group, WIN group, and PDTC group were increased, the cell gaps were reduced, the arrangements were more orderly, and the histopathological changes were improved. Compared with the WIN group, the number of neurons in the hippocampus of the WIN + PDTC group increased, the cell gaps were reduced, and the histopathology improved (Figure 3A,B). The results of Nissl staining showed that the number of neurons in the CUMS group was significantly reduced, and a large number of Nissl bodies disappeared when compared with the control group. The degree of neuronal damage in the Flu group, WIN group, and PDTC group decreased, and the number of the neurons and Nissl corpuscles increased significantly when compared with the CUMS group. The number of neurons and Nissl corpuscles of the WIN + PDTC group increased significantly when compared with the WIN group (Figure 3C,D). The results suggested that WIN55212-2 had a significant protective effect on hippocampal neuron damage caused by CUMS.
WIN55212-2 reduced the inflammatory response of the hippocampus induced by CUMS
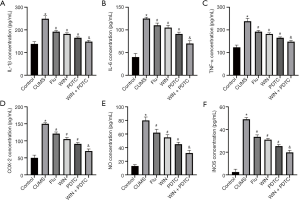
The ELISA test results of rat serum showed that the levels of IL-1β, IL-6, TNF-α, and COX-2 in the hippocampus of the CUMS group were significantly increased when compared with the control group. The levels of IL-1β, IL-6, TNF-α, and COX-2 in the hippocampus of the Flu group, WIN group, and PDTC group were significantly reduced when compared with the CUMS group. The levels of IL-1β, IL-6, TNF-α, and COX-2 in the hippocampus of the WIN + PDTC group were significantly reduced when compared with the WIN group (Figure 4A,B,C,D). The results of biochemical tests and qRT-PCR showed that the expression levels of nitric oxide (NO) and iNOS in the hippocampus of the CUMS group were significantly increased compared with the control group. The expression levels of NO and iNOS in the hippocampus of the Flu group, WIN group, and PDTC group were significantly reduced compared with the CUMS group. The expression levels of NO and iNOS in the hippocampus of the WIN + PDTC group were significantly reduced compared with the WIN group (Figure 4E,F). These results confirmed that WIN55212-2 alleviated the inflammatory response of the hippocampus induced by CUMS.
WIN55212-2 reduced the oxidative stress of the hippocampus caused by CUMS
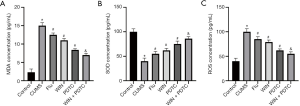
Subsequently, we also tested the corresponding indicators of oxidative stress injury in the tissues. The biochemical test results showed that the levels of NO, malondialdehyde (MDA), and reactive oxygen species (ROS) in the hippocampus of the CUMS group were significantly increased, and the level of superoxide dismutase (SOD) was significantly reduced when compared with the control group. The levels of NO, MDA, and ROS in the hippocampus of the Flu group, WIN group, and PDTC group were significantly reduced, and the level of SOD was significantly increased when compared with the CUMS group. The levels of NO, MDA, and ROS in the hippocampus of the WIN + PDTC group were significantly reduced, and the level of SOD was significantly increased when compared with the WIN group (Figure 5A,B,C). These results showed that WIN55212-2 alleviated the oxidative stress of the hippocampus caused by CUMS.
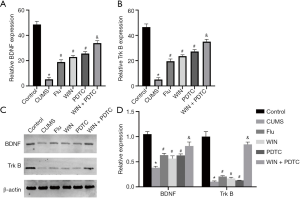
WIN55212-2 reversed the reduced levels of BDNF and TrkB in the hippocampus caused by CUMS
The results of qRT-PCR and western blot showed that the mRNA and protein expression levels of BDNF and TrkB in the hippocampus of the CUMS group were significantly decreased when compared with the control group. The mRNA and protein expression levels of BDNF and TrkB in the hippocampus of the Flu group, WIN group, and PDTC group increased significantly when compared with the CUMS group. The mRNA and protein expression levels of BDNF and TrkB in the hippocampus of the WIN + PDTC group increased significantly when compared with the WIN group (Figure 6A,B,C,D).
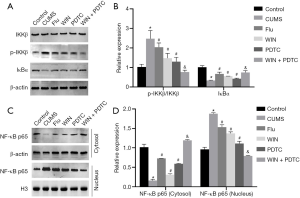
Effects of WIN55212-2 on NF-κB signal transduction in the hippocampus of CUMS rats
Since the NF-κB signaling pathway plays an extremely important role in neuroinflammation and stress responses, we used western blot to detect related proteins (IKKβ, p-IKKβ, IκBα). The results showed that compared with the control group, the expression level of p-IKKβ in the hippocampus of the CUMS group was significantly increased, the expression level of IκBα was significantly decreased, there was no significant difference in the expression level of IKKβ, and the ratio of p-IKKβ/IKKβ was significantly increased. Compared with the CUMS group, the expression level of p-IKKβ in the hippocampus of the Flu group, WIN group, and PDTC group was significantly reduced, the expression level of IκBα was significantly increased, there was no significant difference in the expression level of IKKβ, and the ratio of p-IKKβ/IKKβ was significantly decreased. Compared with the WIN group, the expression level of p-IKKβ in the hippocampus of the WIN + PDTC group was significantly reduced, the expression level of IκBα was significantly increased, there was no significant difference in the expression level of IKKβ, and the ratio of p-IKKβ/IKKβ was significantly decreased (Figure 7A,B). In order to further clarify the effects on its transcription, studies on the NF-κB p65 level in the cytoplasm and nucleus found that compared with the control group, the expression level of NF-κB p65 (cytoplasm) in the hippocampus of the CUMS group was significantly reduced, and the expression level of NF-κB p65 (nucleus) was significantly increased. Compared with the CUMS group, the expression level of NF-κB p65 (cytoplasm) in the Flu group, WIN group, and PDTC group was significantly increased, while the expression level of NF-κB p65 (nucleus) was significantly reduced. Compared with the WIN group, the expression level of NF-κB p65 (cytoplasm) in the WIN+PDTC group was significantly increased, while the expression level of NF-κB p65 (nucleus) was significantly reduced (Figure 7C,D). The above results suggested that WIN55212-2 significantly blocked the activation of the NF-κB signaling pathway.
Discussion
In this study, we found that the CB1R agonist WIN55212-2 can alleviate the anxiety and depressive behaviors of rats induced by CUMS, accompanied by significant changes in the levels of central hippocampal neuroinflammatory cytokines. CUMS is a recognized animal model of depression, which has been shown to cause dysfunction of glucocerebrosidase 1 (GBA) (26). In this study, after 4 weeks of CUMS intervention, weight changes and a series of behavioral experiments (OFT and FST) confirmed that CUMS had successfully induced a mouse model of depression. Rats in the CUMS group showed obvious depressive behaviors, while applying fluoxetine treatment could reduce the depressive behaviors induced by CUMS. At the same time, the CB1R agonist WIN55212-2 and the PDTC also improved the situation of depression and nerve damage induced by CUMS. Compared with fluoxetine, there was no significant difference in the antidepressant effects of WIN55212-2 and PDTC. However, compared with the WIN group, the WIN + PDTC group had better antidepressant effects. The influence of WIN55212-2 on mood and depressive behavior in this study was consistent with previous studies. We further explored the effect of WIN55212-2 on CUMS-induced neuroinflammation in depressive rats. Through qRT-PCR and western blot analysis of hippocampal tissue, we found that WIN55212-2 could significantly inhibit neuroinflammation induced by CUMS, mainly by inhibiting the activation of the NF-κB signaling pathway to delay the pathological process of depression in rats.
The current basic research on depression mainly focuses on the occurrence of neuroinflammation in specific brain regions when the body is stimulated by external stress. Existing evidence shows that serum pro-inflammatory cytokine levels (IL-1β, IL-6, IL-8, IL-12, TNF-α) in patients with depression are higher than normal, but after 3 months of antidepressant treatment, pro-inflammatory cytokine levels can return to normal (5). In addition, a large number of studies have also confirmed that the occurrence of depression is closely related to oxidative stress. The normal oxidative stress level mainly refers to the relative balance of oxidants and antioxidants in the body (27). MDA is a reliable indicator that reflects the metabolic status of oxygen radicals in the body and the degree of tissue attack by free radicals (28), which can also indirectly reflect the degree of neuronal damage (29). SOD has the effects of scavenging free radicals and anti-lipid peroxidation, and a decrease in its activity reflects the decreased ability of the body’s antioxidant defense (30). Both NO and ROS are indicators of oxidative stress injury. The results of this study showed that compared with the normal control group, the levels of NO, MDA, and ROS in the hippocampus of the CUMS group were significantly increased, and the level of SOD was significantly reduced. The results showed that depression had a more obvious oxidative stress response, producing superoxide free radicals and triggering lipid peroxidation, causing attacks from MDA to biomembranes, and destroying the antioxidant defense capability, which is consistent with existing reports. Compared with the CUMS group, the levels of NO, MDA, ROS, and iNOS in the hippocampus of the WIN55212-2 intervention group were significantly reduced, while SOD was significantly increased. This finding suggests that WIN55212-2 can regulate the effects of the oxidative stress response on CUMS-induced rat neuron injury, reducing the production of ROS.
BDNF, a protein with neurotrophic effects (31), plays an important role in the neurodevelopment of the brain (32). Stress or glucocorticoid exposure can down-regulate the expression levels of the hippocampus and prefrontal cortex, and a post-mortem autopsy of depression patients also found that the level of BDNF was reduced in their brains (33). Studies have shown that rats without BDNF exhibit increased sensitivity to stress stimuli, which is sufficient to induce depressive behavior (34). In addition, the combination of BDNF with its receptor, TrkB, can activate a series of downstream signaling pathways related to synapses, which promotes the synthesis of synaptic proteins and increases the positive effects of glutamate receptor circulation on synapse maturation and synapse stability (35). This study suggests that WIN55212-2 may increase the level of BDNF in the hippocampus of patients with depression, and its antidepressant effect may be achieved by increasing the levels of BDNF and TrkB in the hippocampus to protect brain neurons.
We have confirmed that the CB1R agonist WIN55212-2 can significantly inhibit the CUMS-induced depressive behavior of rats by reducing neuroinflammation and oxidative stress. But what is the specific molecular mechanism? A large number of studies have confirmed that the inflammatory response induced by the NF-κB signaling pathway is involved in the occurrence of depression (36). As an important transcription factor in the neuroinflammatory signaling pathway, when activating factors (inflammatory factors, chemokines, iNOS, etc.) combine with the receptors on the cell membrane, NF-κB generates the degradation of IκB caused by proteases and releases NF-κB dimers into the cell nucleus to bind to genes, starting the transcription (37). As a primary transcription factor, NF-κB can rapidly respond to harmful stimuli and activate the expression of pro-inflammatory and oxidative stress cytokines (38). In this study, we confirmed that WIN55212-2 can significantly block the activation of the NF-κB signaling pathway in rats exposed to CUMS. We also confirmed that PDTC, an inhibitor of this signaling pathway, can significantly inhibit CUMS-induced depressive behavior in rats as well as the neuroinflammatory oxidative stress response. The effects are more significant if WIN and PDTC are applied at the same time. The above results suggest that the protective effect of CB1R agonists on depressive rats is mainly based on blocking the NF-κB signaling pathway.
In summary, it was found in this study that the CB1R agonist WIN55212-2 can inhibit neuroinflammation and oxidative stress responses by blocking the NF-κB signaling pathway, thereby reducing depressive behavior and nerve damage in CUMS rats. However, since this study only detected monoamine transmitters in the hippocampus, it cannot fully represent the situation of central monoamine transmitters. Additionally, we only examined CUMS-induced animal models rather than clinical findings. Thus, how the CB1R agonist WIN55212-2 exerts a comprehensive and multi-target regulatory effect on the nervous, immune, and endocrine systems is still unclear, and further research is required.
Acknowledgments
Funding: Nantong Science and Technology Development Plan Project (MS12020026).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://dx.doi.org/10.21037/atm-21-3143
Data Sharing Statement: Available at https://dx.doi.org/10.21037/atm-21-3143
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-3143). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. C202106-8) granted by the Ethics Committee of Guangdong Medical Experimental Animal Center, in compliance with Chinese guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pacchiarotti I, Kotzalidis GD, Murru A, et al. Mixed features in depression: the unmet needs of diagnostic and statistical manual of mental disorders fifth edition. Psychiatr Clin North Am 2020;43:59-68.
- Zhang S, Das-Munshi J, Thornicroft G. Safeguarding the physical health of people with severe mental disorders during the COVID-19 pandemic. BJPsych Bull 2020;44:223-4. [Crossref] [PubMed]
- Cage E, Di Monaco J, Newell V. Experiences of autism acceptance and mental health in autistic adults. J Autism Dev Disord 2018;48:473-84. [Crossref] [PubMed]
- Gaynes BN, Jackson WC, Rorie KD. Major depressive disorder in the primary care setting: strategies to achieve remission and recovery. J Fam Pract 2015;64:S4-15. [PubMed]
- Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017;16:163-80. Erratum in: World Psychiatry 2018;17:120. [Crossref] [PubMed]
- Capuron L, Lasselin J, Castanon N. Role of adiposity-driven inflammation in depressive morbidity. Neuropsychopharmacology 2017;42:115-28. [Crossref] [PubMed]
- Teicher MH, Samson JA. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry 2016;57:241-66. [Crossref] [PubMed]
- Murgatroyd CA, Peña CJ, Podda G, et al. Early life social stress induced changes in depression and anxiety associated neural pathways which are correlated with impaired maternal care. Neuropeptides 2015;52:103-11. [Crossref] [PubMed]
- Wang HT, Huang FL, Hu ZL, et al. Early-life social isolation-induced depressive-like behavior in rats results in microglial activation and neuronal histone methylation that are mitigated by minocycline. Neurotox Res 2017;31:505-20. [Crossref] [PubMed]
- Zhang HX, Xu YQ, Li YY, et al. Difference in proinflammatory cytokines produced by monocytes between patients with major depressive disorder and healthy controls. J Affect Disord 2018;234:305-10. [Crossref] [PubMed]
- Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord 2013;150:736-44. [Crossref] [PubMed]
- Dahl J, Ormstad H, Aass HC, et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 2014;45:77-86. [Crossref] [PubMed]
- Jiang H, Wang Z, Wang Y, et al. Antidepressant-like effects of curcumin in chronic mild stress of rats: involvement of its anti-inflammatory action. Prog Neuropsychopharmacol Biol Psychiatry 2013;47:33-9. [Crossref] [PubMed]
- Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry 2011;35:664-75. [Crossref] [PubMed]
- Kendall DA, Yudowski GA. Cannabinoid receptors in the central nervous system: their signaling and roles in disease. Front Cell Neurosci 2017;10:294. [Crossref] [PubMed]
- Lu HC, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry 2016;79:516-25. [Crossref] [PubMed]
- Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci 2018;19:833. [Crossref] [PubMed]
- Selvam R, Yeh ML, Levine ES. Endogenous cannabinoids mediate the effect of BDNF at CA1 inhibitory synapses in the hippocampus. Synapse 2018; Epub ahead of print. [Crossref] [PubMed]
- Guo S, Liu Y, Ma R, et al. Neuroprotective effect of endogenous cannabinoids on ischemic brain injury induced by the excess microglia-mediated inflammation. Am J Transl Res 2016;8:2631-40. [PubMed]
- Laprairie RB, Bagher AM, Denovan-Wright EM. Cannabinoid receptor ligand bias: implications in the central nervous system. Curr Opin Pharmacol 2017;32:32-43. [Crossref] [PubMed]
- Wang F, Han J, Higashimori H, et al. Long-term depression induced by endogenous cannabinoids produces neuroprotection via astroglial CB1R after stroke in rodents. J Cereb Blood Flow Metab 2019;39:1122-37. [Crossref] [PubMed]
- Poleszak E, Wośko S, Sławińska K, et al. Cannabinoids in depressive disorders. Life Sci 2018;213:18-24. [Crossref] [PubMed]
- Wang M, Dong W, Wang R, et al. Gastrodiae Rhizoma water extract ameliorates hypothalamic-pituitary-adrenal axis hyperactivity and inflammation induced by chronic unpredictable mild stress in rats. Biomed Res Int 2020;2020:8374614 [Crossref] [PubMed]
- Tsatsakis AM, Docea AO, Calina D, et al. Hormetic neurobehavioral effects of low dose toxic chemical mixtures in real-life risk simulation (RLRS) in rats. Food Chem Toxicol 2019;125:141-9. [Crossref] [PubMed]
- Biedermann SV, Biedermann DG, Wenzlaff F, et al. An elevated plus-maze in mixed reality for studying human anxiety-related behavior. BMC Biol 2017;15:125. [Crossref] [PubMed]
- Burokas A, Arboleya S, Moloney RD, et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry 2017;82:472-87. [Crossref] [PubMed]
- Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem 2017;86:715-48. [Crossref] [PubMed]
- Amin MM, Rafiei N, Poursafa P, et al. Association of benzene exposure with insulin resistance, SOD, and MDA as markers of oxidative stress in children and adolescents. Environ Sci Pollut Res Int 2018;25:34046-52. [Crossref] [PubMed]
- Senchuk MM, Dues DJ, Van Raamsdonk JM. Measuring oxidative stress in Caenorhabditis elegans: paraquat and juglone sensitivity assays. Bio Protoc 2017;7:e2086 [Crossref] [PubMed]
- Filograna R, Godena VK, Sanchez-Martinez A, et al. Superoxide dismutase (SOD)-mimetic M40403 is protective in cell and fly models of paraquat toxicity: implications for Parkinson disease. J Biol Chem 2016;291:9257-67. [Crossref] [PubMed]
- Han W, Zhang C, Wang H, et al. Alterations of irisin, adropin, preptin and BDNF concentrations in coronary heart disease patients comorbid with depression. Ann Transl Med 2019;7:298. [Crossref] [PubMed]
- Hui J, Yan Z, Liang F, et al. Dynamic association of plasma brain-derived neurotrophic factor, neuron-specific enolase, and S100β with delirium in critically ill patients. Chinese Journal of Emergency Medicine 2018;27:1132-5.
- Phillips C. Brain-derived neurotrophic factor, depression, and physical activity: making the neuroplastic connection. Neural Plast 2017;2017:7260130 [Crossref] [PubMed]
- Yu H, Wang DD, Wang Y, et al. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci 2012;32:4092-101. [Crossref] [PubMed]
- Collingridge GL, Peineau S, Howland JG, et al. Long-term depression in the CNS. Nat Rev Neurosci 2010;11:459-73. [Crossref] [PubMed]
- Du RH, Tan J, Sun XY, et al. Fluoxetine inhibits NLRP3 inflammasome activation: implication in depression. Int J Neuropsychopharmacol 2016;19:pyw037 [Crossref] [PubMed]
- Nazari M, Khodadadi H, Fathalizadeh J, et al. Defective NF-kB transcription factor as the mediator of inflammatory responses: a study on depressed Iranian medical students. Clin Lab 2013;59:827-30. [Crossref] [PubMed]
- Tsai CY, Li FC, Wu CH, et al. Sumoylation of IkB attenuates NF-kB-induced nitrosative stress at rostral ventrolateral medulla and cardiovascular depression in experimental brain death. J Biomed Sci 2016;23:65. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


