Evaluation of the reporting quality of clinical practice guidelines on prostate cancer using the RIGHT checklist
Introduction
Prostate cancer is the most commonly diagnosed cancer among males across the word (1). The Global Cancer Observatory estimated that there were nearly 1.3 million new cases of prostate cancer and 360,000 prostate cancer related deaths in 2018 worldwide. Prostate cancer is expected to become the most common type of cancer by 2030, with one in eight men expected to be diagnosed with prostate cancer during their lifetime (2). Despite the fact that prostate cancer is the second leading cause of cancer-related death in males, survival rates have greatly improved over the past four decades with the earlier diagnosis through Prostate-Specific Antigen (PSA) testing and new treatments. The prostate cancer incidence rates declined approximately 6.5% per year from 2007 and after years of significant decline (from 1993 to 2013), the overall prostate cancer mortality trend stabilized in recent years (3,4).
Clinical practice guidelines (CPGs) are important means of guiding medical personnel to make decisions based on the latest scientific evidence (5). In recent years, an increasing amount of academic organizations and institutions have developed CPGs related to prostate cancer (6). Clear and transparent reporting is an important factor that can facilitate effective dissemination and implementation of the guidelines (7). The reporting quality of CPGs in many fields has been shown to be heterogeneous (8).
Reporting Items for Practice Guidelines in Healthcare (RIGHT) (9) is a checklist that aims to assure a high quality of reporting. RIGHT is endorsed by the World Health Organization (WHO) in its guideline development manual. This study aims to evaluate the reporting quality of CPGs for prostate cancer using the RIGHT tool.
Methods
Literature search
We systematically searched Medline (via PubMed), Wanfang, China Biology Medicine (CBM) and China Knowledge Network (CNKI) databases, and the websites of the World Health Organization (WHO, https://www.who.int/publications/guidelines/year/en/), The National Institute for Health and Care Excellence (NICE, https://www.nice.org.uk/), National Comprehensive Cancer Network (NCCN, https://www.nccn.org/), Scottish Intercollegiate Guidelines The Scottish Intercollegiate Guidelines Network (SIGN, https://www.sign.ac.uk/our-guidelines/), Guidelines International Network (GIN, https://guidelines.ebmportal.com/), to identify all published CPGs on prostate cancer. The search time was limited to the time period from January 1, 2018 to December 1, 2020. The main search terms were “Prostatic Neoplasm”, “Prostate Cancer”, “Prostatic Cancer”, “Prostatic tumor” and “Prostate tumor”.
Inclusion and exclusion criteria
Two investigators (KF Liu and YF Ma) screened all titles and abstracts of the retrieved records to identify potentially relevant studies. In the next step, the full texts of the selected articles were screened by two investigators independently to decide about inclusion. Disagreements were resolved through discussion or by consulting a senior investigator (YJ Yang). Articles were included if they met the following inclusion criteria: (I) the article was a CPG on testing, diagnosis, treatment, or management of prostate cancer; and (II) the language of publication was Chinese or English. We excluded translations, summaries and interpretations of guidelines, as well as draft or unpublished guidelines.
Data extraction
We designed a data extraction table based on the RIGHT checklist, and the two trained investigators extracted the information from the included guidelines independently. Disagreements were solved by discussion. We extracted the basic information of the included guidelines, including the title, publication year, country or region of development, topic, developer agency, system used to grade the quality of evidence, the impact factor (IF) of the journal, and the funding source.
Reporting quality assessment using the RIGHT checklist
The RIGHT checklist consists of 35 items, which grouped into seven domains: basic information, background, evidence, recommendations, review and quality assurance, financial support and declaration and management of conflicts of interests, and other information. Each item was evaluated by both reviewers (KF Liu and YF Ma) independently as either “reported” (relevant information was sufficiently reported) or "not reported" (some relevant information was lacking). If an item did not apply for a specific guideline, we rated it as “not applicable”. Before the formal evaluation, two reviewers were trained to use the RIGHT tool, and two rounds of pre-tests were completed to ensure that the reviewers understood each item consistently. Disagreements were resolved through consensus or consulting with a third reviewer (YJ Yang).
Statistical analysis
We calculated the number and percentage of guidelines reporting each item, and conversely the percentage of items reported by each guideline. We also present the mean proportions of reported items for each RIGHT domain and overall. We also present the mean overall reporting rates stratified by the year of publication, language, type of developer organization, country or region of origin, and reporting of funding.
Results
Identification of guidelines
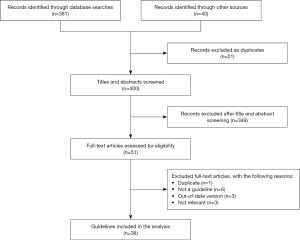
Our initial search retrieved 421 records, including 339 in English and 82 in Chinese. After screening the titles and abstracts, and the full texts of articles deemed potentially eligible, 38 articles were included into this study. The screening process and results are presented in Figure 1.
Characteristics of selected guidelines
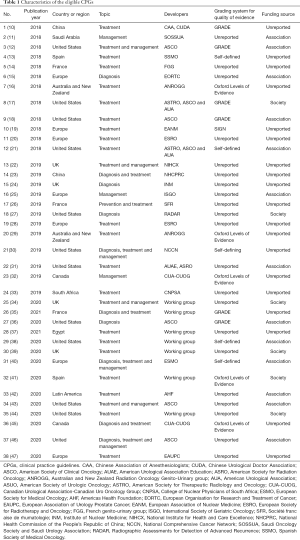
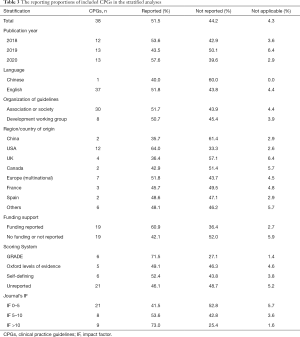
Table 1 shows the basic characteristics of the included articles. Twelve guidelines were published in 2018, 13 in 2019 and 13 in 2020. Thirty-seven were published in English and one in Chinese. The included guidelines addressed the diagnosis, treatment and management of prostate cancer. Two guidelines were developed in China, twelve in the United States, seven by European multinational collaborations, four in the United Kingdom, three in France, two in Spain, two in Canada, two in Australia and New Zealand, one in Egypt, one in South Africa, one in Saudi Arabia and one by a multinational collaboration from Latin America. Eighteen guidelines were supported by government or institutional funds, and twenty did not report their funding sources.
Full table
Overall analysis of reporting quality
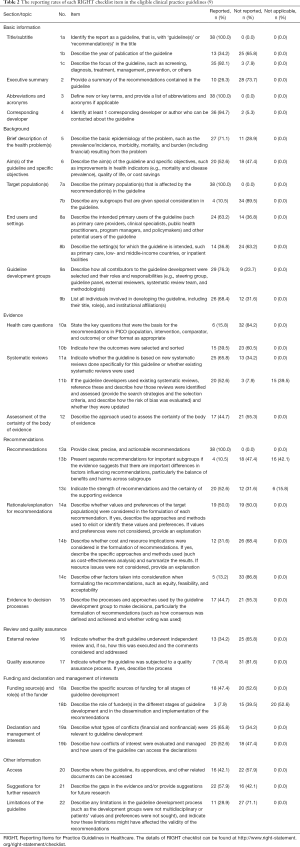
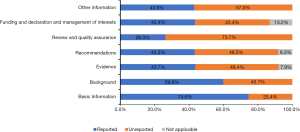
The mean reporting rate of all 35 RIGHT checklist items in the included guidelines was 51.6%. Eighteen items were reported by more than half of the guidelines Items 1a, 3, 7a and 13a were reported by all guidelines (Table 2). Items 7b (reporting rate 10.5%), 13b (10.5%), 14c (13.2%), and 18b (7.9%), had the lowest reporting rates. Among the domains of the RIGHT checklist, “Basic Information” had the highest mean reporting rate (74.6%), and “Review and quality assurance” the lowest rate (26.3%). The mean reporting rates in the remaining domains were 59.9% for “Background”, 43.7% for “Evidence”, 43.25 for “Recommendations”, 43.4% for “Funding and declaration and management of interests”, and 43.0% for “Other information” (Figure 2).
Full table
Stratified analyses of reporting quality
The mean overall reporting proportion improved slightly over time, being 53.6% in guidelines published in 2018, 43.5% in 2019, and 57.6% in 2020 (Table 3). Guidelines published in Chinese had a reporting rate of 40.0%; for guidelines published in English the reporting rate was 51.8%. Guidelines that reported their funding sources had a higher reporting rate (60.9%) than those that either did not report funding, or reported receiving no funding (42.1%).
Full table
Discussion
This study evaluated the reporting quality of 38 CPGs for prostate cancer using the RIGHT checklist. The mean overall compliance to the items of RIGHT was 51.6%. Twenty-two of the 38 guidelines adhered to less than half of the items. Items related to basic information and background were relatively well reported, whereas the compliance with items related to the review and quality assurance was poor. In particular the description of patient groups that need special consideration and the role of the funders were extremely rarely reported.
The domains for basic information and background had a relatively high reporting rate across all guidelines. However, the publication year and a summary of the recommendations tended to be poorly reported. Of the top 50% of guidelines ranked by overall reporting proportions, only four reported the year of publication in the title, and 74% of them did not have a summary. Most guidelines included in our review had deficiencies in reporting the review and quality assurance process. Items pertaining to external review, peer reviewers, review process and management of the feedback were poorly reported.
Guidelines with funding support had a higher overall reporting quality than those not declaring or having funding. Funding is of great importance, because the development, maintenance, effective dissemination and implementation of guidelines is an expensive and labor-intensive endeavor (48). CPG panels with financial support may confer quality benefits. However, few guidelines reported the sources of funding for the different stages of the development, dissemination and implementation of the guideline and recommendations in detail. The results suggest a need for greater transparency and rigor in the role of funders.
We found several factors correlated with the reporting quality of guidelines. First, the reporting quality tended to be higher in journals with a high IF, which could reflect more rigorous editorial policies and peer review. Second, high reporting integrity was found, as expected, in guidelines that have targeted and sufficient funding. Guideline development is expensive and time consuming, so adequate funding or resources can promote guideline quality (49). Third, guidelines that use a grading system of evidence, such as GRADE, tended to comply better with RIGHT. The use of a grading system may reflect a high level of methodological experience or knowledge, or attending specific training in guideline methodology, by the development team.
To our knowledge, our study is the first time that the RIGHT checklist were used to evaluate the reporting quality of clinical practice guidelines on prostate cancer. However, we also has several limitations. The included CPGs were heterogeneous in many aspects, which may explain the observed differences in reporting integrity. The stratified presentation of the reporting quality does not capture the impact of all factors and the interactions between them. We only included guidelines published in Chinese and English.
Questions to be further discussed and considered
Question 1: What impact do you think the low reporting quality of clinical practice guidelines on prostate cancer will have on clinicians and clinical practices?
Expert opinion: Dr. Francesco Del Giudice
Prostate Cancer Guidelines have a terrific impact on the clinical practice of thousands of urologists worldwide. The diagnostic and therapeutic choices of many practitioners is definitively and strongly influenced form the Guidelines therefore an overall low quality of these Guidelines might generate confusion and disorientation in the clinical daily practice.
Expert opinion: Dr. Masaki Shiota
Clinical practice guidelines are expected to be used by clinicians in clinical practice to help improve the quality of care and avoid unnecessary care. Low quality reporting of clinical practice guidelines can lead to various problems. First, it may hinder the dissemination of clinical practice guidelines because they do not gain the trust from clinicians. In addition, inadequate content may lead to inappropriate medical care. Finally, low reporting quality of clinical practice guidelines on prostate cancer may prevent improvements of disease understanding, quality of care, patient outcomes, and efficient medical care.
Expert opinion: Dr. Shingo Hatakeyama
It is no doubt that the low reporting quality of clinical practice guidelines on prostate cancer has a great impact on clinical practice.
However, the meaning of “low quality” needs further discussion.
If the “low quality” means “old evidence”, it needs revision as soon as possible. However, a very rapid paradigm shift based on many Phase III RCT makes it difficult to change clinical practice guidelines quickly. We realized that the updated clinical practice guideline of 2020 become the “old one” in 2021. Although the update is necessary, update every year is not easy task.
If the “low quality” means “low compliance to evidence”, it is not suitable for a clinical practice guideline. The experience-based recommendation needs to revise to the robust evidence from Phase III RCT. However, medical situation (such as insurance system, cost, etc.) needs to take this into account in each country.
If the “low quality” means “too many conflicts of interest”, it is a difficult situation. Statement of COI needs to open.
Question 2: What impact does the low reporting quality of clinical practice guidelines on prostate cancer have on clinicians and clinical practices?
Expert opinion: Dr. Francesco Del Giudice
Increasing the Quality level of PCa Guidelines can be obtained by: (I) adoption and clinical utilization of practical diagnostic and therapeutic algorithms; (II) more clarity in the level of recommendation for each single item not only in terms of Level of Evidence but also (and importantly) on the Level of Adoption in daily practice; (III) greater amount of cost/benefits analysis.
Expert opinion: Dr. Masaki Shiota
The purpose of the RIGHT checklist is to assist guideline development. Similarly, the Appraisal of Guidelines for Research & Evaluation II (AGREE II) is another tool that was developed to assess the rigor and transparency of methods in the guideline development process. Therefore, the use of RIGHT checklists and AGREE II will enable the development of high-quality guidelines for clinical practice guidelines on prostate cancer.
Expert opinion: Dr. Shingo Hatakeyama
The answer is simple. We need to update it every year! But hard task for us.
Question 3: How do you think conflicts of interest in the guidelines should be handled?
Expert opinion: Dr. Francesco Del Giudice
All conflicts of interest of each single Guideline panel member should be highlighted at the beginning of the guidelines specifying accurately the relationship between the scientific societies and the commercial companies enrolled in the products (pharmaceuticals, surgical devices etc.) that take place in the everyday clinical management of PCa.
Expert opinion: Dr. Masaki Shiota
Appropriate reporting of financial and non-financial interests and their implications in guideline development is important for transparent and high-quality guidelines. Therefore, the development needs to address these concerns. Therefore, it is necessary to describe the potential financial and non-financial conflicts of interest of guideline authors. As well, it should clearly state how conflicts of interest were assessed and managed.
Expert opinion: Dr. Shingo Hatakeyama
We could not avoid the influence of COI. We need to open COIs.
Conclusions
The overall adherence of CPGs on prostate cancer to RIGHT checklist is poor. The reporting of some aspects, such as quality assurance, executive summary, subgroups, funding sources and the role of the funder, need particular attention. Developers of prostate cancer CPGs are advised to improve the completeness of reporting and take advantage of tools such as the RIGHT checklist to promote the dissemination and implementation of their guidelines.
Acknowledgments
The authors appreciate the academic support from the AME Reporting Guideline Collaborative Group.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/atm-21-2956). MS received research funding support from Daiichi Sankyo Company, Ltd., and honoraria from Janssen Pharmaceutical K.K., AstraZeneca K.K., and Astellas Pharma Inc. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018;103:356-87. [Crossref] [PubMed]
- Negoita S, Feuer EJ, Mariotto A, et al. Annual Report to the Nation on the Status of Cancer, part II: Recent changes in prostate cancer trends and disease characteristics. Cancer 2018;124:2801-14. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Institute of Medicine. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press 2011. Available online:
10.17226/13058 10.17226/13058 - Hebert D, Lindsay MP, McIntyre A, et al. Canadian stroke best practice recommendations: Stroke rehabilitation practice guidelines, update 2015. Int J Stroke 2016;11:459-84. [Crossref] [PubMed]
- Van der Wees PJ, Hendriks EJ, Custers JW, et al. Comparison of international guideline programs to evaluate and update the Dutch program for clinical guideline development in physical therapy. BMC Health Serv Res 2007;7:191. [Crossref] [PubMed]
- Grilli R, Magrini N, Penna A, et al. Practice guidelines developed by specialty societies: the need for a critical appraisal. Lancet 2000;355:103-6. [Crossref] [PubMed]
- Chen Y, Yang K, Marušic A, et al. A Reporting Tool for Practice Guidelines in Health Care: The RIGHT Statement. Ann Intern Med 2017;166:128-32. [Crossref] [PubMed]
- Chinese Association of Anesthesiologists, Chinese Urological Doctor Association. ERAS Chinese Expert and Pathway Management Expert (2018): The surgical part of prostate cancer treatment. J Mod Urol 2018;12:902-9.
- Aljubran A, Abusamra A, Alkhateeb S, et al. Saudi Oncology Society and Saudi Urology Association combined clinical management guidelines for prostate cancer 2017. Urol Ann 2018;10:138-45. [Crossref] [PubMed]
- Bekelman JE, Rumble RB, Chen RC, et al. Clinically Localized Prostate Cancer: ASCO Clinical Practice Guideline Endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology Guideline. J Clin Oncol 2018;36:3251-8. [Crossref] [PubMed]
- Cassinello J, Arranz JÁ, Piulats JM, et al. SEOM clinical guidelines for the treatment of metastatic prostate cancer (2017). Clin Transl Oncol 2018;20:57-68. [Crossref] [PubMed]
- Langrand-Escure J, de Crevoisier R, Llagostera C, et al. Dose constraints for moderate hypofractionated radiotherapy for prostate cancer: The French genito-urinary group (GETUG) recommendations. Cancer Radiother 2018;22:193-8. [Crossref] [PubMed]
- Lecouvet FE, Oprea-Lager DE, Liu Y, et al. Use of modern imaging methods to facilitate trials of metastasis-directed therapy for oligometastatic disease in prostate cancer: a consensus recommendation from the EORTC Imaging Group. Lancet Oncol 2018;19:e534-45. [Crossref] [PubMed]
- Lieng H, Hayden AJ, Christie DRH, et al. Radiotherapy for recurrent prostate cancer: 2018 Recommendations of the Australian and New Zealand Radiation Oncology Genito-Urinary group. Radiother Oncol 2018;129:377-86. [Crossref] [PubMed]
- Morgan SC, Hoffman K, Loblaw DA, et al. Hypofractionated Radiation Therapy for Localized Prostate Cancer: Executive Summary of an ASTRO, ASCO, and AUA Evidence-Based Guideline. Pract Radiat Oncol 2018;8:354-60. [Crossref] [PubMed]
- Morris MJ, Rumble RB, Basch E, et al. Optimizing Anticancer Therapy in Metastatic Non-Castrate Prostate Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1521-39. [Crossref] [PubMed]
- Poeppel TD, Handkiewicz-Junak D, Andreeff M, et al. EANM guideline for radionuclide therapy with radium-223 of metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2018;45:824-45. [Crossref] [PubMed]
- Salembier C, Villeirs G, De Bari B, et al. ESTRO ACROP consensus guideline on CT- and MRI-based target volume delineation for primary radiation therapy of localized prostate cancer. Radiother Oncol 2018;127:49-61. [Crossref] [PubMed]
- Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J Urol 2018;199:683-90. [Crossref] [PubMed]
- NICE Guideline Updates Team (UK). Prostate cancer: diagnosis and management. London: National Institute for Health and Care Excellence (UK) 2019. Available online: https://www.nice.org.uk/guidance/ng131
- National Health Commission Of The People's Republic Of China. Chinese guidelines for diagnosis and treatment of cervical cancer 2018 (English version). Chin J Cancer Res 2019;31:295-305. [Crossref] [PubMed]
- Afaq A, Gleeson F, Scarsbrook A, et al. UK guidelines on 18F-fluciclovine PET/CT in prostate cancer imaging. Nucl Med Commun 2019;40:662-74. [Crossref] [PubMed]
- Boyle HJ, Alibhai S, Decoster L, et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur J Cancer 2019;116:116-36. [Crossref] [PubMed]
- Briot K, Paccou J, Beuzeboc P, et al. French recommendations for osteoporosis prevention and treatment in patients with prostate cancer treated by androgen deprivation. Joint Bone Spine 2019;86:21-8. [Crossref] [PubMed]
- Crawford ED, Koo PJ, Shore N, et al. A Clinician's Guide to Next Generation Imaging in Patients With Advanced Prostate Cancer (RADAR III). J Urol 2019;201:682-92. [Crossref] [PubMed]
- Ghadjar P, Fiorino C, Munck Af Rosenschöld P, et al. ESTRO ACROP consensus guideline on the use of image guided radiation therapy for localized prostate cancer. Radiother Oncol 2019;141:5-13. [Crossref] [PubMed]
- Lieng H, Kneebone A, Hayden AJ, et al. Radiotherapy for node-positive prostate cancer: 2019 Recommendations of the Australian and New Zealand Radiation Oncology Genito-Urinary group. Radiother Oncol 2019;140:68-75. [Crossref] [PubMed]
- Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:479-505. [Crossref] [PubMed]
- Pisansky TM, Thompson IM, Valicenti RK, et al. Adjuvant and Salvage Radiotherapy after Prostatectomy: ASTRO/AUA Guideline Amendment 2018-2019. J Urol 2019;202:533-8. [Crossref] [PubMed]
- Saad F, Aprikian A, Finelli A, et al. 2019 Canadian Urological Association (CUA)-Canadian Uro Oncology Group (CUOG) guideline: Management of castration-resistant prostate cancer (CRPC). Can Urol Assoc J 2019;13:307-14. [Crossref] [PubMed]
- Vorster M, Warwick J, Lawal IO, et al. South African guidelines for receptor radioligand therapy (RLT) with Lu-177-PSMA in prostate cancer. S Afr J Surg 2019;57:45-51. [Crossref] [PubMed]
- Brown JE, Handforth C, Compston JE, et al. Guidance for the assessment and management of prostate cancer treatment-induced bone loss. A consensus position statement from an expert group. J Bone Oncol 2020;25:100311 [Crossref] [PubMed]
- Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2021;79:243-62. [Crossref] [PubMed]
- Eggener SE, Rumble RB, Armstrong AJ, et al. Molecular Biomarkers in Localized Prostate Cancer: ASCO Guideline. J Clin Oncol 2020;38:1474-94. [Crossref] [PubMed]
- Elghazaly H, Mottet N, Garcia J, et al. Clinical recommendations in the management of advanced prostate cancer: International Gastrointestinal, Liver and Uro-oncology (IGILUC 2019) experts. World J Urol 2021;39:1421-9. [Crossref] [PubMed]
- Lowrance W, Breau R, Chou R, et al. Adavanced prostate cancer: AUA/ASTRO/SUO guideline. American Urological Association 2020. Available online: https://www.auanet.org/guidelines/guidelines/advanced-prostate-cancer
- O'Sullivan JM, Carles J, Cathomas R, et al. Radium-223 Within the Evolving Treatment Options for Metastatic Castration-resistant Prostate Cancer: Recommendations from a European Expert Working Group. Eur Urol Oncol 2020;3:455-63. [Crossref] [PubMed]
- Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:1119-34. [Crossref] [PubMed]
- Puente J, Anido U, Climent MÁ, et al. Expert recommendations on the management of patients with metastatic castration-resistant prostate cancer who progress after CHAARTED or LATITUDE. Ther Adv Med Oncol 2020;12:1758835920920067 [Crossref] [PubMed]
- Reis RBD, Alías-Melgar A, Martínez-Cornelio A, et al. Prostate Cancer in Latin America: Challenges and Recommendations. Cancer Control 2020;27:1073274820915720 [Crossref] [PubMed]
- Saylor PJ, Rumble RB, Tagawa S, et al. Bone Health and Bone-Targeted Therapies for Prostate Cancer: ASCO Endorsement of a Cancer Care Ontario Guideline. J Clin Oncol 2020;38:1736-43. [Crossref] [PubMed]
- Shore ND, Antonarakis ES, Cookson MS, et al. Optimizing the role of androgen deprivation therapy in advanced prostate cancer: Challenges beyond the guidelines. Prostate 2020;80:527-44. [Crossref] [PubMed]
- So AI, Chi KN, Danielson B, et al. Canadian Urological Association-Canadian Urologic Oncology Group guideline on metastatic castration-naive and castration-sensitive prostate cancer. Can Urol Assoc J 2020;14:17-23. [PubMed]
- Trabulsi EJ, Rumble RB, Jadvar H, et al. Optimum Imaging Strategies for Advanced Prostate Cancer: ASCO Guideline. J Clin Oncol 2020;38:1963-96. [Crossref] [PubMed]
- Van den Broeck T, van den Bergh RCN, Briers E, et al. Biochemical Recurrence in Prostate Cancer: The European Association of Urology Prostate Cancer Guidelines Panel Recommendations. Eur Urol Focus 2020;6:231-4. [Crossref] [PubMed]
- Boyd EA, Akl EA, Baumann M, et al. Guideline funding and conflicts of interest: article 4 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report. Proc Am Thorac Soc 2012;9:234-42. [Crossref] [PubMed]
- McCaul M, Ernstzen D, Temmingh H, et al. Clinical practice guideline adaptation methods in resource-constrained settings: four case studies from South Africa. BMJ Evid Based Med 2020;25:193-8. [Crossref] [PubMed]