
VEGF gene transfection restores the angiogenesis of oral submucous fibrosis in mice
Introduction
Areca is a plant of the palm family that is widely cultivated in tropical Asia. Betel-quid (BQ) is a mixture of areca nut (AN) in combination with betel leaf and slaked lime (1). It can make consumers feel euphoric, fight fatigue, increase salivation, and achieve satiety (2). As a result, chewing betel nut is enjoyed by people all over the world, especially in India, Taiwan, and southeast Asia. There are an estimated 600 million BQ users worldwide (1).
Oral submucous fibrosis (OSF) is a chronic, insidious, and progressive disease. The incidence of OSF is mainly concentrated in tropical and subtropical regions. India has the highest incidence of OSF, followed by Taiwan and other regions (3). AN, a highly addictive drug, has been identified as the major etiological factor of OSF (4). Arecoline is the most important active constituent of AN. The exact mechanism of OSF is not clear yet. But the arecoline and flavonoid, components of areca nut when exposed to buccal mucosal fibroblast results in the accumulation of collagen. Reduced collagenase activity and increased cross-linking of the fibers results in decreased degradation of collagen (5). Early OSMF shows thickened epithelium with rete ridges and varying degrees of inflammation and vascularity. The epithelium is atrophic with subepithelial hyalinization and dense fibrosis evidenced in the connective tissue of middle and advanced OSMF. In an early experiment (6), we developed a BALB/c mouse model of OSF induced by arecoline. Arecoline is a major arecoline alkaloid, which can stimulate the synthesis of collagen in normal buccal mucosa fibroblasts (BMFs) and promote the formation of OSF (7). These changes initially affect the lamina propria, and gradually, the submucosa and the musculature, resulting in vascular occlusion and loss of tissue elasticity. Currently, the treatments of OSF (8) mainly include habit control (that is, stop eating betel nuts), drug therapy, surgical intervention, and physical therapy. However, there are some adverse effects, such as poor drug permeability, and greater surgical trauma and recurrence rates. Drug therapy (9) mainly includes vasodilators. Studies have shown (10) that vasodilators can improve mouth opening in approximately 62% of patients with OSF. However, due to the use of systemic drugs, its side effects and targeting are not satisfactory. Therefore, finding an effective and reliable treatment is one of the focuses of our research. VEGF is a key angiogenic stimulator (11). Several studies (12) have shown that VEGF can promote angiogenesis and the development of collateral vessels in experimental models of animal limb ischemia. Sharma et al. (13) found that there was a significant increase in vascularity in early OSF. With the progression of OSF, VEGF expression was found to be down-regulated. This suggests that it is possible to improve the symptoms of oral fibrosis by vascular reconstruction. In recent years, gene therapy has been widely studied. For the past 2 decades, it was common for researchers to use viral vectors, particularly adenovirus vectors (14,15). Here, we aimed to assess whether VEGF restores the angiogenesis of OSF in mice. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-21-2213).
Methods
Ethical permission
Experiments were performed under a project license (No. 201511031) granted by the board of Committee of the Guangxi Medical University Laboratory Animal Center, in compliance with Chinese guidelines for the care and use of animals. A protocol was prepared before the study without registration.
Animals and reagents
BALB/c male mice (4 weeks old) were obtained from the Experimental Animal of Beijing Weitong Lihua Limited Company, license: SCXK Beijing 2012-0001. The animals were housed under controlled conditions, including 24 h alternate light and a room with a relative humidity level of 30–50%. The mice were fed with standard mouse fodder. Arecoline (Sigma, USA) was diluted to a concentration of 1,000 mg/L in the drinking water for the experimental group and distilled water for the control group. The mice were allowed to drink water, which was replaced once a week, and were fed the chow diet ad libitum at all times. Animal groups: In the transfection experiment, 40 mice were divided into the experimental group and the control group, and 10 mice in the control group drank distilled water freely while the other 30 mice in the experimental group drank the arecoline solution freely. The rest of the mouse samples were all from the mouse OSF model constructed by our research group (6).
Mouse fibroblast isolation, culture, and identification
Under general anesthesia, normal mice were fixed in a supine position on the operation table, the buccal mucosa tissue was removed under aseptic conditions, rinsed with cold PBS solution 3 times, and soaked with biclonal antibody and amphotericin for 5 min. The tissue was cut into pieces of about 1 mm3 and put into a culture bottle, then placed into an incubator for 10 minutes. A small amount of DMEM medium was added, containing 20% new-born calf serum, which was replaced with fresh medium 3 days later. The second generation adherent cells were digested by trypsin (2.5 g/L) for 1 min, and the digestion was terminated with the addition of new-born calf serum (20%). The concentration of suspended cells was adjusted to a density of 2×106/mL. The cells were seeded into 6-well plates which had sterile slides. Following 3–5 days of culture, the cells were identified by immunochemical staining (Vimentin antibody was purchased from Boster, Wuhan, China).
Fibroblast transfection with Ad-EGFP-VEGF165
The second generation of fibroblasts were transfected with Ad-EGFP-VEGF165 (Gi Kai Technologies, China), and post-transfection, the cells were incubated at 37 °C in an atmosphere containing 5% CO2. Three multiplicities of infection (MOI: 20, 50, and 100) were used, and cellular proliferation was assessed using the MTT assay. Following experimentation, the fibroblasts were transfected with the different MOIs of Ad-EGFP-VEGF165 for 24, 48, and 72 h, respectively, and the transfection efficiency was measured by fluorescence microscopy (Olympus Optical Co., Ltd., Tokyo, Japan).
Analysis of the protein expression levels of VEGF by ELISA
On days 1, 2, 3, 4, 5, 6, and 7 following gene transfer, the Ad-EGFP-VEGF165/fibroblasts, Ad-EGFP/fibroblasts, and non/fibroblasts were transfected, and the supernatants were collected. The expression of VEGF was detected by ELISA (Shanghai Yuanye Biologic Products, Inc., Shanghai, China) with the supernatants as the samples.
Quantitative real-time PCR
The fibroblasts and mouse buccal tissues were collected and the total RNA was extracted. Reverse transcription of 2 mg of total RNA was performed using a high-capacity cDNA synthesis kit (Takara, Japan). PCR reactions were performed in conjunction with a gene-specific primer pair. The normalizing gene was glyceraldehyde-3-phosphate dehydrogenase (G3PDH). The product size and primer sequences were as follows: VEGF165(148bp) GCGGATCAAACCTCACCAAG (forward) and GCTTTCGTTTTTGCCCCTTTC (reverse). GAPDH (80 bp) GGTTGTCTCCTGCGACTTCA (forward) and TGGTCCAGGGTTTCTTACTCC (reverse). The reaction was performed in a 7500 Real-Time PCR System (Life Technologies) using SYBR Premix Ex Taq (Takara, Japan) according to the manufacturer’s protocol. All calculated concentrations of the target genes were divided by the amount of the endogenous reference G3PDH to obtain the normalized VEGF expression values. The reactions were performed in triplicate for each sample. All of the primers used in the study provided a single peak in the dissociation curve, suggesting that there was a single amplicon.
Immunohistochemistry and scoring (CD34 and VEGF)
Paraffin sections were subjected to immunohistochemical staining. Peroxidase activity was quenched by treatment with 0.2% H2O2 for 3 h. The sections were incubated with mono-clonal CD34 and VEGF (ZSGB-Bio, China), overnight at 4 °C. Immunostaining was performed using SP kit (ZSGB-Bio, China). the DAB solution was added, and sections were observed under the microscope to control the color development time (within 2 min). Sections were then dehydrated transparently, sealed with neutral gum, and observed. To assess CD34 expression in OSF, all morphological structures with cavities surrounded by CD34 positive endothelial cells were considered microvessels. Vascular endothelial cells were stained brown (cytoplasmic expression), alone or in clusters that were distinctly separate from adjacent microvessels. Vessels of the muscular layer were not counted. The highest density of staining was measured by a preliminary scan at 100× magnification in the OSF tissue, and 3 areas were randomly selected under high power (400×). The number of blood vessels positive for CD34 expression was evaluated by 2 independent observers. To evaluate the immunohistochemical expression of CD34, the mean of the 3 fields was taken as the final score.
Hematoxylin and eosin (HE), Van Gieson (VG), and Masson staining of oral mucosal lesion specimens (containing partial deep muscle tissue)
A portion of the tissue sample was fixed in 10% formalin, embedded in paraffin, sectioned into 4 mm sections, and stained with HE. VG staining was used to stain collagen fibres and Masson’s trichrome was used to stain for newly synthesized collagen.
Statistical analysis
SPSS version 17.0 was used for statistical analyses. The data are presented as the mean ± SEM. The evaluation of statistical significance was determined by a one-way analysis of variance (ANOVA) followed by multiple comparison tests. For all tests, a P value of less than 0.05 was considered to be significant.
Results
AD-EGFP-VEGF165 transfection increased the expression of VEGF in mouse fibroblasts
Cultured fibroblasts were transfected with different amounts (20, 50, 100 U) of Ad-VEGF recombinant adenovirus or control adenovirus for different periods of time (24, 48, 72 h). The recombinant Ad-VEGF adenovirus was marked with the GFP protein, therefore, the VEGF-GFP protein expression in fibroblasts was also assessed by fluorescence microscopy. When the MOI =100, the efficiency of fluorescence transfection was the highest after transfection for 72 h (Figure 1A). There were no significant differences in the green fluorescence expression efficiency between the AD-EGFP-VEGF165 group and the AD-EGFP group (P>0. 05) (Figure 1B). Using 100 U of Ad-VEGF adenovirus, fibroblasts were transfected for 72 h, and the results of the real-time quantitative RT-PCR assay showed that the VEGF expression level was dramatically higher compared with other groups (Figure 1C). The mouse fibroblasts were used for immunohistochemistry, and the Ad-EGFP-VEGF165 group had positive expression of VEGF while the other groups had negative expression (Figure 1D). The results of the ELISA assay for VEGF expression also confirmed this result (Figure 1E). VEGF expression (1–7 days) in fibroblasts transfected with Ad-EGFP-VEGF165 peaked at 6 days and then reduced from 7 days (Figure 1E).
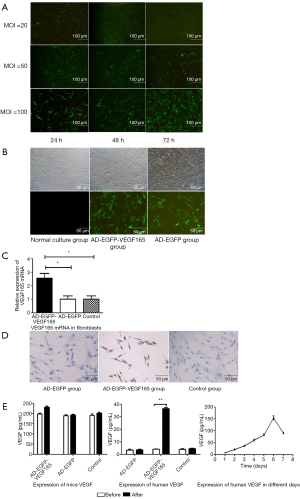
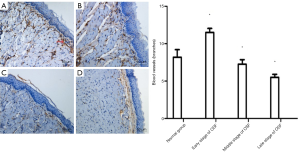
The number of blood vessels in different stages of OSF mice and the histopathological changes in the late stage of OSF
The number of blood vessels in the oral mucosa increased in the early stage of OSF compared with the normal oral mucosa and the middle and late stages of OSF, while the number of blood vessels in the oral mucosa began to decrease in the middle stage of OSF (Figure 2). In the late stage of OSF, the number of oral mucosal vessels was (5.50±0.41), which was significantly decreased from the early stage (11.50±0.54) and the middle stage (7.25±0.41) compared with the normal group (8.20±1.01) (P<0.05).
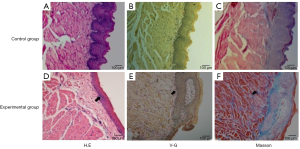
Development of a mouse model of arecoline-induced oral mucosal fibrosis
The mouse OSF model was constructed based on previous experiments (6). We fed mice for 20 weeks and collected the buccal tissue. The HE staining mainly showed atrophy of buccal mucosal epithelium, gradual disappearance or thinning of epithelial nail processes, thickened lamina propria, infiltration of inflammatory cells, and reduction of lamina propria blood vessels compared with control mice. The VG staining mainly showed that more collagen was stained in red and collagen accumulation and formation of collagen fibrous bands could be seen in the lamina propria of buccal mucosa compared with control mice. Masson staining showed that there was more collagen stained with blue and purple, and these blue-purple fibers were mainly distributed in the lamina propria of buccal mucosa compared with control mice (Figure 3).
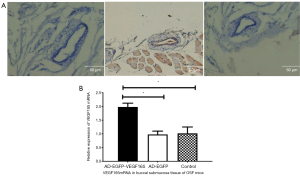
VEGF gene-transfected buccal submucosal tissue had increased VEGF expression in OSF mice
The buccal submucosal tissue was transfected with 100 U of Ad-VEGF recombinant adenovirus or control adenovirus for 6 days. The buccal submucosal tissue of OSF mice were obtained for immunohistochemistry. The Ad-EGFP-VEGF165 group had positive expression of VEGF while the other groups had negative expression (Figure 4A). The results of the real-time quantitative RT-PCR assay showed that the VEGF expression level was dramatically higher compared with other groups (Figure 4B).
VEGF gene-transfected buccal submucosal tissue had an increased number of blood vessels in OSF mice
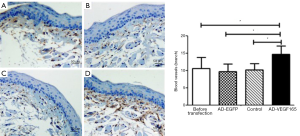
The buccal submucosal tissue was transfected with 100 U of Ad-VEGF recombinant adenovirus or control adenovirus for 6 days. In the AD-EGFP-VEGF165 group, it was observed that the amount of positive staining and beam spots were greater than the other groups (Figure 5A: before transfection group, Figure 5B: AD-EGFP group, Figure 5C: saline group, Figure 5D: AD-EGFP-VEGF165 group. The number of blood vessels in submucosal tissue of the AD-EGFP-VEGF165 group was more than that in the other groups, and the difference was statistically significant (P<0.05).
Discussion
In the early stage of Oral Submucous Fibrosis (OSMF), The underlying submucosal fibrosis in OSMF causes obliteration of the blood vessels and hypoxia of the tissues, leading to the induction of hypoxia inducible factor-1α (HIF-1α) and angiogenesis through upregulation of vascular endothelial growth factor (VEGF). Research findings of Gupta et al. (16): the mean serologic levels of HIF-1α, VEGF-A, and VEGF-C were significantly raised in patients with OSMF compared with healthy controls (P<0.001).
Several studies (17) have suggested that the underlying cause of the pathogenesis of OSF is the progressive loss of vascularity in the diseased mucosa. The decrease of blood vessels may lead to the accumulation of locally formed collagen that cannot be metabolized through blood circulation, which causes tissue ischemia and hypoxia, and ultimately promotes the formation and development of fibrosis. VEGF is an effective direct vascular inducing factor, can promote the proliferation and differentiation of vascular endothelial cells, angiogenesis, improve vascular permeability and induce vascular dilation. TSP is an important anti-vascular growth factor, which can inhibit angiogenesis, promote platelet adhesion and thrombosis, and promote fibrosis. Studies (18) have shown that the expression of VEGF and the number of blood vessels increase in the early stage of human OSF lesions. The main reason is (19), during the course of the Oral submucous fibrosis, accumulation of inflammatory cells along with fibrosis can lead to hypoxia. In hypoxia, hypoxia-inducible factor 1 (HIF-1) induces angiogenesis. As newly formed vessels are immature and vulnerable, correction of the ischemic state of fibrosis could become difficult. This sets in persistent hypoxia resulting in the continuous production of proinflammatory and angiogenic molecules. These can eventually stimulate extracellular matrix (ECM) deposition and progression of fibrosis. Thus, the inhibition of pathological angiogenesis is of great significance in the prevention and treatment of oral submucosal fibrosis (20). In the advanced stage, the expression of VEGF and the number of blood vessels decrease, and the area of the vascular cavity shrinks in the later stages of OSF. The expression of TSP increased continuously in the early and middle stages of OSF, and slightly decreased in the advanced stage, with no significant difference among all groups. VEGF was negatively correlated with TSP in the submucosa of the diseased tissue (21). The abnormal expression of VEGF and TSP is closely related to OSF microvascular disease, which suggests that it is possible to find a way to promote VEGF secretion or inhibit the production of TSP to improve the microcirculation, so as to provide a new method for the treatment of OSF. VEGF165 is the dominant secretion form in the human body. It is reported that the local application of exogenous VEGF can significantly enhance the survival of ischemic flaps (22). In our study, we demonstrated that AD-EGFP-VEGF165 can be successfully transfected into mouse fibroblasts and mouse cheek tissues. In cell experiments, we found that the expression of mouse VEGF and human VEGF increased after transfection of mouse fibroblasts with AD-EGFP-VEGF165. Li et al. (23). showed that after transfection of human VEGF165 plasmid into fibroblasts, the concentration of VEGF164 in cells transfected with the VEGF165 gene was higher than that in untransfected cells. This may indicate that the exon structure of VEGF164 is similar to human VEGF165.
In our study, the OSF mouse model was constructed based on previous experiments (6). We fed mice for 20 weeks and collected the buccal tissue. We found buccal epithelial atrophy and changes in which spikes disappeared or flattened, as well as the accumulation of collagen fibers. Furthermore, the number of vascular lesions decreased in the middle and late stages of OSF. This pathological change is the same as the change in oral mucosa fibrosis in humans. At present, VEGF has been used extensively to promote neovascularization in ischemic tissues. VEGF has been delivered either as a recombinant protein or plasmid and as viral gene constructs to human subjects in an attempt to improve lower limb perfusion. So called “therapeutic angiogenesis” has also been used to treat ischemic heart disease (24,25). This suggests that angiogenesis can be restored in the middle and late stages of OSF by transfection of VEGF. Barr et al. (26) successfully transfected cardiomyocytes with adenovirus vectors and found that the target genes peaked at the first week after transfection, gradually decreased, and disappeared after about 4–5 weeks. This is similar to the dynamic change of VEGF concentration in the supernatants 1–7 days after after we transfected fibroblasts. In our cell study, there was high expression of VEGF on day 6 after transfection. Therefore, we collected buccal tissues of OSF mice 6 days after transfection. The vital signs of mice were observed daily and weights were measured daily after injection. No significant changes were observed. This indicated that in the submucosal tissues of the buccal mucosa of adenovirus-transfected mice, transfection did not cause significant damage to mice in the short term.
Many oral squamous cell carcinomas are preceded by clinically evident potentially malignant oral disorders such as hyperkeratosis or epithelial hyperplasia, epithelial dysplasia, erythroplakia, and OSMF. OSMF is characterized by in ammation and progressive mucosal fibrosis (27). Epithelial changes include hyperplasia in the early stage and atrophy in the advanced stage. Angiogenesis also plays an important role in tumor progression and there is increasing evidence that the process of angiogenesis commences in the premalignant stages of most cancers, serving as an alternative marker of tumor development. However, the majority of cases do not progress to malignancy. So, it is difficult to determine the occurrence of tumor just from the changes in the number of blood vessels and the changes in the mucosa. Histological features of oral submucous fibrosis include juxta-epithelial fibrosis, hyalinized collagen accumulation beneath the basement membrane and progressive reduction of vascularity (28). The measurement of vascularity cannot be done directly, but quantification can be done indirectly by means of angiogenic markers such as CD34, VEGF. VEGF can carry signals by combining with specific receptors on the surface of endothelial cells, so as to maintain normal blood vessel integrity, increase vascular permeability, and promote endothelial proliferation and angiogenesis (29). CD34 is a type of vascular regeneration epithelial marker. Pandiar and Shameena (30) evaluated the immunoreactivity of CD34 in different histological grades of OSF. Mean vascular density was found to decrease significantly as the disease advanced. In our study, we found that the expression levels of CD34 and VEGF in the AD-EGFP-VEGF group were higher than those in the other groups (P<0.05), and there were more blood vessels in the lamina propria of buccal tissue. This demonstrated that the VEGF gene can be successfully transfected into the buccal mucosa of mice, and can restore the angiogenesis of OSF in mice. In summary, our study forms the foundation for a preliminary clinical trial on the restoration of OSF angiogenesis via AD-EGFP-VEGF.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81460105), the National Natural Science Foundation of China (Grant No. 81960199), the National Natural Science Foundation of Hainan Province (Grant No.819MS115) and Supported by the Key Specialty Program of Hainan Provincial Clinical Medical Center in 2021.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-21-2213
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-21-2213
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-21-2213). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 201511031) granted by the board of Committee of the Guangxi Medical University Laboratory Animal Center, in compliance with Chinese guidelines for the care and use of animals. A protocol was prepared before the study without registration.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee CH, Ko MS, Warnakulasuriya S, et al. Intercountry prevalences and practices of betel-quid use in south, southeast and eastern Asia regions and associated oral preneoplastic disorders: an international collaborative study by Asian betel-quid consortium of south and east Asia. Int J Cancer 2011;129:1741-51. [Crossref] [PubMed]
- Aziz SR. Coming to America Betel nut and oral submucous fibrosis. J Am Dent Assoc 2010;141:423-8. [Crossref] [PubMed]
- Shih YH, Wang TH, Shieh TM, et al. Oral Submucous Fibrosis: A Review on Etiopathogenesis, Diagnosis, and Therapy. Int J Mol Sci 2019;20:1-22. [Crossref] [PubMed]
- Angadi P V, Rao SS. Areca nut in pathogenesis of oral submucous fibrosis: revisited. Oral Maxillofac Surg 2011;15:1-9. [Crossref] [PubMed]
- Yoithapprabhunath TR, Maheswaran T, Dineshshankar J, et al. Pathogenesis and therapeutic intervention of oral submucous fibrosis. Journal of Pharmacy & Bioallied Sciences 2013;5:S85-8. [Crossref] [PubMed]
- Wen QT, Wang T, Yu DH, et al. Development of a mice model of arecoline- induced oral mucosal fibrosis. Asian Pac J Trop Med 2017;10:1177-84. [Crossref] [PubMed]
- Sar JI, Yang CJ, Tsai YS, et al. Sphingosine-1-phosphate stimulated connective tissue growth factor expression in human buccal fibroblasts: Inhibition by epigallocatechin-3-gallate. J Formos Med Assoc 2015;114:860-4. [Crossref] [PubMed]
- Saman W, Kerr AR. Oral submucous fibrosis: a review of the current management and possible directions for novel therapies. Oral Surg Oral Med Oral Pathol Oral Radiol 2016;122:232-41. [Crossref] [PubMed]
- Chole RH, Gondivkar SM, Gadbail AR, et al. Review of drug treatment of oral submucous fibrosis. Oral Oncol 2012;48:393-8. [Crossref] [PubMed]
- Bhadage CJ, Umarji HR, Shah K, et al. Vasodilator isoxsuprine alleviates symptoms of oral submucous fibrosis. Clin Oral Investig 2013;17:1375-82. [Crossref] [PubMed]
- Patel KR, Vajaria BN, Begum R, et al. VEGFA isoforms play a vital role in oral cancer progression. Tumour Biol 2015;36:6321-32. [Crossref] [PubMed]
- Wang C, Zhang B, Lin Y, et al. Effects of Adenovirus-mediated VEGF165 Gene Therapy on Myocardial Infarction. Ann Clin Lab Sci 2018;48:208-15. [PubMed]
- Sharma E, Tyagi N, Gupta V, et al. Role of angiogenesis in oral submucous fibrosis using vascular endothelial growth factor and CD34: An immunohistochemical study. Indian J Dent Res 2019;30:755-62. [Crossref] [PubMed]
- Schultz BR, Chamberlain JS. Recombinant Adeno-associated Virus Transduction and Integration. Mol Ther 2008;16:1189-99. [Crossref] [PubMed]
- Lee CS, Bishop ES, Zhang R, et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis 2017;4:43-63. [Crossref] [PubMed]
- Gupta SR, Sharma A, Gupta N, et al. Single nucleotide polymorphisms and serologic levels of hypoxia-inducible factor1 α and vascular endothelial growth factor are associated with increased risk of oral submucous fibrosis in gutka users among a North Indian population. Oral Surg Oral Med Oral Pathol Oral Radiol 2020;130:557-64. [Crossref] [PubMed]
- Rajendran R, Rani V, Shaikh S. Pentoxifylline therapy: A new adjunct in the treatment of oral submucous fibrosis. Indian J Dent Res 2006;17:190. [Crossref] [PubMed]
- Zhang C, Meng C, Guan D, et al. BMP2 and VEGF165 transfection to bone marrow stromal stem cells regulate osteogenic potential in vitro. Medicine 2018;97:e9787 [Crossref] [PubMed]
- Park S, Kim J W, Kim J H, et al. Differential Roles of Angiogenesis in the Induction of Fibrogenesis and the Resolution of Fibrosis in Liver. Biol Pharm Bull 2015;38:980-5. [Crossref] [PubMed]
- Choudhari SS, Kulkarni DG, Patankar S, et al. Angiogenesis and Fibrogenesis in Oral Submucous Fibrosis: A Viewpoint. J Contemp Dent Pract 2018;19:242-5. [Crossref] [PubMed]
- Wang J, Zhu J, Peng J, et al. The expression and correlation between VEGF and TSP in oral submucous fibrosis. Journal of Practical Stomatology 2009;25:513-6.
- Solovyeva VV, Chulpanova DS, Tazetdinova LG, et al. In Vitro Angiogenic Properties of Plasmid DNA Encoding SDF-1α and VEGF165 Genes. Appl Biochem Biotechnol 2020;190:773-88. [Crossref] [PubMed]
- Li R, Li CA, Mckee MD, et al. Effect of human vascular endothelial growth factor gene transfer on endogenous vascular endothelial growth factor mRNA expression in a rat fibroblast and osteoblast culture model. J Orthop Trauma 2010;24:547-51. [Crossref] [PubMed]
- Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation 2004;109:2487-91. [Crossref] [PubMed]
- Modarai B, Humphries J, Burnand KG, et al. Adenovirus-mediated VEGF gene therapy enhances venous thrombus recanalization and resolution. Arterioscler Thromb Vasc Biol 2008;28:1753-9. [Crossref] [PubMed]
- Barr E, Carroll J, Kalynych AM, et al. Efficient catheter-mediated gene transfer into the heart using replication-defective adenovirus. Gene Ther 1994;1:51-8. [PubMed]
- Murgod VV, Kale AD, Angadi PV, et al. Morphometric analysis of the mucosal vasculature in oral submucous fibrosis and its comparison with oral squamous cell carcinoma. J Oral Sci 2014;56:173-8. [Crossref] [PubMed]
- Sharma E, Tyagi N, Gupta V, et al. Role of angiogenesis in oral submucous fibrosis using vascular endothelial growth factor and CD34: An immunohistochemical study. Indian J Dent Res 2019;30:755-62. [Crossref] [PubMed]
- Wang T, Liao TA, Zhong SB. Transfection of bone marrow mesenchymal stem cells using green fluorescence protein labeled hVEGF165 recombinant plasmid mediated by liposome. Asian Pac J Trop Med 2013;6:739-42. [Crossref] [PubMed]
- Pandiar D, Shameena P. Immunohistochemical expression of CD34 and basic fibroblast growth factor (bFGF) in oral submucous fibrosis. J Oral Maxillofac Pathol 2014;18:155-61. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


