Sixty shades of oxygen—an attractive opportunity for cancer immunotherapy
Tumors evade the cytotoxic activity of T lymphocytes by secreting adenosine in the extracellular matrix which limits the infiltration and activation of cytotoxic T lymphocytes (CTLs), and this process is strongly fostered by the hypoxic microenvironment that characterizes most of the cancer types. A paper recently published in Science Translational Medicine by Hatfield et al. (1) found that respiratory hyperoxygenation can promote tumor regression in preclinical models of cancer by re-activating the antitumoral function of CTLs and natural killer (NK) cells.
When solid tumors reach a certain size, some areas do not receive enough nutrients and oxygen to meet their energy demand. Such regions, hence termed hypoxic regions, force a change in cell metabolism and foster secretion of many cytokines and chemokines in the tumor microenvironment (TME), altogether allowing adaptation to oxygen shortage and sustaining tumor progression. These metabolic and microenvironmental changes promote the production of adenosine, that, in tumor tissues as well as in inflamed tissues, inhibits the action of CTLs through an A2A adenosine receptor-mediated signal (2). Such a signal limits CTL trafficking and initiates immunosuppressive mechanisms (Figure 1).
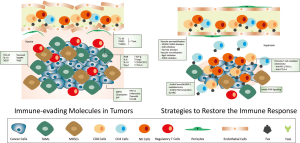
Hatfield and colleagues (1) report that the weakly-immunogenic fibrosacroma and melanoma cells, injected intravenously, showed growth regression when recipient mice were exposed to 60% oxygen compared to control mice breathing at a normal atmosphere, containing 21% oxygen. This regression required the combined action of both T cell subtypes, namely CD4+ T helper cells and CD8+ CTLs but, mostly, of NK cells. To further prove their point, Hatfield et al. showed that γc/Rag-2−/− mice with genetic T and NK cell deficiency did not show any tumor regression upon exposure to hyperoxia.
Interestingly, this switch from an immunosuppressive to an immunopermissive TME was characterized by an increased infiltration of CD8+ cells into the tumor and a decrease of hypoxic CD8+ cells. Also, oxygen breathing led to increased release of proinflammatory mediators and a simultaneous decrease of immunosuppressive cytokines. However, how and whether oxygen affects directly NK cells was not reported, suggesting the indirect involvement of other tumor cell compartments on NK infiltration.
T cells and NK cells are known to be capable of destroying target tumor cells through multifaceted and complex pathways (3). In order for T cells to reach the tumor bed and exert their cytotoxic effect, they have to overcome a hostile multi-factorial environment. Cancer cells down-regulate the major histocompatibility complex (MHC) molecules while they up-regulate the inhibitory receptor programmed death-ligand 1 (PD-L1). T cells are also faced by a consortium of immunosuppressive cells that include regulatory T cells (Treg), tumor-associated macrophages (TAMs), and myeloid derived suppressor cells (MDSCs) together with the inhibitory factors they produce such as interleukin 10 (IL-10), IL-6, arginase 1 (Arg-1), and transforming growth factor-β (TGF-β). Added to that, T cells encounter various inhibitory metabolites as adenosine and a decrease of tryptophan levels in a medium of low pH. Most importantly, CTLs and NK cells are physically and functionally not allowed to cross the tumor vasculature towards the tumor bed (4). Hypoxic areas of tumors up-regulate semaphorin 3A (Sema3A) which recruits macrophages by interacting with neuropilin 1 (NRP1) that drives an attraction signal through PlexinA1/PlexinA4/vascular endothelial growth factor receptor 1 (VEGFR1) holoreceptor. This attraction mechanism is inverted into a stop action when macrophages enter the hypoxic areas as they down-regulate NRP1 levels (5). Hypoxic TAMs secrete several immunosuppressive and proangiogenic factors as CC-chemokine ligand 22 (CCL22), IL-10, VEGF, Sema3A, and matrix metalloprotease 9 (MMP9). When TAMs’ entry to the hypoxic areas of tumors is prevented, the immune response is restored with consequent rejection of the tumor. In an alternative study (6), histidine-rich glycoprotein (HRG) was found to promote tumor regression and to inhibit metastasis by blocking the production of placental growth factor (PlGF) by TAMs. HRG exerted its action by skewing TAMs towards the proinflammatory M1 phenotype and subsequent secretion of immune-stimulating cytokines that support dendritic cell (DC) and CTL-mediated immune responses in addition to supporting vessel normalization, thus increasing chemotherapeutic drug delivery to the tumor.
What is even more encouraging in the work proposed by Hatfield et al. is that hyperoxia could weaken immune-suppression due to decreased infiltration of Treg in the TME, decreased forkhead box P3 (Foxp3) expression, reduced levels of the adenosine-producing CD39/CD73 enzymes, and a decreased cytotoxic T lymphocyte-associated protein 4 (CTLA-4) levels. Consistently with a switch towards a pro-immune TME, upon adoptive-transfer of tumor-reactive T cells or NK cells to tumor-bearing mice, tumor regression in the hyperoxic environment was enhanced, with a stronger effect shown by NK cells. The combination of hyperoxia with CTLA-4 or programmed death-1 (PD-1) blockade displayed a synergic effect, encouraging the use of this strategy in future clinical use (Figure 1). As shown in previous studies, especially in human melanoma and ovarian cancer samples, a high CD8+/Treg ratio in tumors is associated with better disease prognosis (7,8). Other approaches that aim at restoring the immune response against tumors were previously reported. For example, Motz et al. (9) describe the role of the death mediator Fas ligand (FasL) in tumor-associated immune-suppression. FasL is solely expressed by the endothelium of tumor vessels, but not of normal tissues, and it induces cell-death in CD8+ and CD4+CD25− cells while Treg are insensitive to such an effect. This is due to the fact that Treg express Fas at low levels, and they also overcome Fas-mediated apoptosis by expressing the anti-apoptotic factor FADD-like IL-1β-converting enzyme-inhibitory protein (c-FLIP). FasL is induced in the endothelium directly by the positive interaction of the hypoxia-mediated factors IL-10 and prostaglandin E2 (PGE2) together with the augmentation of VEGFA. Upon interfering with FasL production or Fas-FasL signaling, a significant tumor regression was observed in several mouse cancer models. Such a regression was due to the disruption of the tumor endothelial physical barrier, which became permissive to the CD8+ T cell infiltration and allowed their mediated anti-tumor activity. This was achieved through the combined use of VEGFA blockers, cyclooxygenase (COX) inhibitors and FasL-specific antibodies.
Facciabene et al. (10) systematically identified CCL28 to be induced by hypoxia; once secreted, CCL28 selectively attracts Treg, which, in hypoxic conditions, increase the levels of VEGFA and support angiogenesis on top of their immunosuppressive role.
Consequently, this shows the importance of having a functional endothelium in order to favor the entry of immunocompetent cells inside the tumor but also to prevent chronic hypoxia. This clarifies the reason why, regardless of initial perception, the use of VEGF/VEGFR blockers as antiangiogenic therapies turned out not to be as effective as expected; after a period of initial tumor regression, angiogenesis and tumor progression restarted again. The reason behind this was not clear until Rivera et al. (11) dissected the molecular mechanisms behind this behavior. The major player in such a resistance was the activation of phosphoinositide 3-kinase (PI3K) signaling in myeloid cells which bypasses the VEGF signal blockade with all the accompanying tumor progression events. In light of previous findings, it became clear that vessel normalization is more clinically relevant than vessel pruning (Figure 1). And this effect can be achieved by different strategies, at least in tumor mouse models. For example, by blocking the regulator of G-protein signaling 5 (Rgs5), tumors are more oxygenated and more permeable to adoptively transferred CD8+ and CD4+ T cells, which increased the survival rates in mouse tumor models (12). Following the same theme, we suggested that inhibition of prolyl hydroxylase 2 (PHD2) gives rise to endothelial quiescence and vessel normalization, leading to less metastases and less invasiveness, which also increased the survival rate in mouse tumor models (13). Also, chemotherapeutic agents could reach their target cells more efficiently while normal organs as heart and kidney were protected (14).
The very intriguing message that the current report is bringing about is that oxygen is all is needed to improve cancer outcome. Nevertheless, reality does not seem to be that simple, there still remain many questions to be answered in that regard. Primarily, it remains unclear how this approach could be successfully used in the clinic in order to treat cancer patients, especially since the therapeutic effect of using hyperoxygenation seems to be dose dependent and it can be either ineffective or even potentially induce an inflammatory reaction. Another concern, also related to the safety of this approach, is the lack of tissue-specificity as it has a generalized effect rather than a localized one. Further studies will be thus required to verify whether using hyperoxygenation as a therapeutic strategy can match the benefits observed in mice, either when used alone or in combination with immune stimulatory agents or even with standard chemotherapeutic and irradiation-based regimens, given the fact that hypoxia provides cancer cells with the mechanisms to escape these treatments (15). Alternatively, a pharmacologic opportunity is represented by those agents that restore the vascular architecture and function within the tumor bed, thus preventing hypoxia.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hatfield SM, Kjaergaard J, Lukashev D, et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci Transl Med 2015;7:277ra-30. [PubMed]
- Ohta A, Gorelik E, Prasad SJ, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA 2006;103:13132-7. [PubMed]
- Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol 2002;2:401-9. [PubMed]
- Lanitis E, Irving M, Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr Opin Immunol 2015;33:55-63. [PubMed]
- Casazza A, Laoui D, Wenes M, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell 2013;24:695-709. [PubMed]
- Rolny C, Mazzone M, Tugues S, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 2011;19:31-44. [PubMed]
- Piras F, Colombari R, Minerba L, et al. The predictive value of CD8, CD4, CD68, and human leukocyte antigen-D-related cells in the prognosis of cutaneous malignant melanoma with vertical growth phase. Cancer 2005;104:1246-54. [PubMed]
- Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 2005;102:18538-43. [PubMed]
- Motz GT, Santoro SP, Wang LP, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med 2014;20:607-15. [PubMed]
- Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 2011;475:226-30. [PubMed]
- Rivera LB, Meyronet D, Hervieu V, et al. Intratumoral myeloid cells regulate responsiveness and resistance to antiangiogenic therapy. Cell Rep 2015;11:577-91. [PubMed]
- Hamzah J, Jugold M, Kiessling F, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature 2008;453:410-4. [PubMed]
- Mazzone M, Dettori D, Leite de Oliveira R, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 2009;136:839-51. [PubMed]
- Leite de Oliveira R, Deschoemaeker S, Henze AT, et al. Gene-targeting of Phd2 improves tumor response to chemotherapy and prevents side-toxicity. Cancer Cell 2012;22:263-77. [PubMed]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011;11:393-410. [PubMed]


