
An ultrasound-derived stroke risk score to identify patients at high risk of stroke
Introduction
Stroke is a leading cause of death and long-term disability worldwide (1). Approximately 15% to 20% of all ischemic strokes are attributable to artery-to-artery thromboembolism caused by carotid atherosclerotic disease (2). Currently, guidelines for prevention on stroke in patients with carotid atherosclerosis are based on quantification of carotid stenosis degree (3,4). However, a growing amount of evidence has shown that vulnerable carotid plaques play an important role in the occurrence of stroke, independent of the severity of carotid stenosis (5,6). This paradigm shift of assessment of the occurrence of ischemic events provides a new strategy for primary and secondary stroke prevention (7).
Carotid ultrasound is the primary noninvasive technique for diagnosing and following up with atherosclerotic carotid disease (8). Conventional ultrasound can evaluate plaque morphology, size, echogenicity, and hemodynamic changes and provide an important basis for clinical decisions. Despite the recent development of several ultrasound techniques, none of them are currently used in clinical routines for carotid plaque screening (9,10). These new techniques, such as shear wave elastography (SWE) (11), enable the assessment of plaque stiffness and may add a new possible surrogate parameter for symptomatic atherosclerotic plaques.
At present, risk stratification of patients with asymptomatic carotid stenosis is still controversial for carotid revascularization. Improved approaches to identify patients at the highest risk of ischemic events may refine clinical decisions, allowing treatment to be more precisely targeted towards patients who are most likely to benefit. The aim of this study was to develop a risk score scale of multiparameter ultrasound, including plaque stiffness, surface morphology and carotid stenosis degree, for the identification of patients at high risk of stroke and to investigate the discrimination performance of the scale. We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/atm-20-8205).
Methods
Study participants
A systematic description of the patient selection, ultrasound evaluation, and clinical information was previously described (12). Patients with >50% proximal internal carotid artery lumen narrowing confirmed by carotid ultrasound were consecutively included at Beijing Tiantan Hospital from December 2018 to December 2019. The exclusion criteria were patients showed a definitive cardioembolic cause of stroke or an unusual etiology according to the TOAST standard (13); unable to cooperate to complete ultrasound evaluation; greater than 70% ipsilateral intracranial artery stenosis confirmed by cerebrovascular imaging; non-atherosclerotic carotid artery stenosis; and poor-quality of ultrasound image. All participants underwent a detailed demographic characteristics assessment by a well-trained study physician. All patients underwent multimode ultrasound evaluation, including B-mode ultrasound, color Doppler ultrasound, spectral Doppler ultrasound, and SWE, on the bilateral carotid arteries before study inclusion. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Review Board approval was obtained to perform this study (IRB No. KY2019-113-01). Informed consent was obtained from all patients.
Clinical outcome
Symptomatic group was defined as patients who had already experienced ipsilateral non-cardioembolic ischemic cerebrovascular events including acute stroke, transient ischemic attack (TIA), and amaurosis fugax within the past 30 days before study inclusion. Asymptomatic group was defined as patients who had showed no symptoms and signs of ischemic events within the past 6 months. All clinical events were collected and confirmed by a well-trained neurologist who was blinded to the ultrasound results.
Routine ultrasound protocol
Routine ultrasound was evaluated using a Canon Aplio 900 (Canon Medical Systems, Japan) ultrasound system, equipped with a 7.5-MHz linear probe. All patients underwent multimode ultrasound examination (including B-mode ultrasound, color Doppler ultrasound, and spectral Doppler ultrasound) on bilateral carotid arteries (including common carotid artery, carotid artery bifurcation, and internal carotid artery). Bilateral carotid arteries were scanned in the longitudinal (anterior, lateral, and posterior views) and transverse planes, with the patient in the supine position. All ultrasound images were collected and analyzed independently by two vascular sonographers who had at least ten years of experience. The vascular sonographers were blinded to the neurologist’s findings.
Based on the peak-systolic and end-diastolic velocities, internal carotid artery stenosis severity was graded as <50%, 50–69% and 70–99% (14). The definition of carotid atherosclerosis plaque was a focal structure thickness greater than 1.5 mm or a distinct area of carotid intima-media thickness (CIMT) ≥50% greater than the surrounding vessel wall. Plaque size (area), echogenicity, surface morphology (smooth, irregular and ulceration), and carotid stenosis severity were collected, respectively. Plaque risk biomarkers were evaluated in longitudinal planes according to clinical consensus (15,16). When the ultrasound image showed the thickest plaque, the CIMT (including the plaque) was taken as the maximal internal carotid plaque thickness (17). And then, a plaque was manually depicted and measured as the plaque size. Plaque echogenicity characteristics were defined according to greyscale ultrasound as follows: uniformly hypoechoic, predominantly hypoechoic, predominantly hyperechoic, and uniformly hyperechoic (18). Plaque ulceration was measured as a defect, at least 2 mm × 2 mm in depth and width, in the plaque with the basal margin echo weaker than the surrounding plaque surface, and on color Doppler ultrasound, the defect was filled with blood flow signals (19).
Plaque elasticity protocol
Plaque elasticity assessment was performed immediately after routine ultrasound evaluation by using the identical ultrasound system to gain a double-view display of the interest plaque in the elastographic diagram and quality control diagram. The probe was placed on the plaque without pressure to keep the image stable to allow measurements of plaque elasticity. Plaques elastography measurements were performed in the maximum longitudinal section for 3 times, and the mean of three independent measurements was used for further analyses. Plaque elasticity was reflected in real-time by means of a chromaticity diagram within the elastographic rectangular sampling frame. The speed of propagation of the shear waves is dependent on the local elastic modulus of the tissue. The shear wave range was set from 0 to 10 m/s. The elastographic-specific region of interest (ROI) frame was modified to contain the whole plaque. Plaque elasticity measurement was depicted around the entire plaque, and the quantitative elasticity of the whole plaque was recorded in m/s. Young’s modulus (YM, kPa) is typically estimated using the equation YM = 3ρc2, where ρ is the density of specific organizations, and c is the shear wave velocity. The quality control diagram displays the propagation of the shear wave as wave-forward lines. When the propagation lines are parallel to each other, the measurement may be more reliable; if the lines are distorted or lacking, the measurement needs to be repeated. A sample (10%) of the SWE findings was selected to assess the interobserver agreement of the two sonographers using the intraclass correlation coefficient (ICC). For this analysis, the elasticity of the selected plaques for each image was measured by the two observers.
Baseline characteristics
A well-trained research neurologist who was blinded to the imaging results collected the demographic characteristic information that may be associated with ischemic cerebrovascular events from all patients. A standard case report form was used to record information, including age, sex, status of smoking and drinking, history of diseases, and medication use.
Statistical analysis
Differences in baseline clinical variables between the symptomatic group and asymptomatic group were compared using nonparametric tests for continuous variables and χ2 tests for categorical variables. Carotid plaque stiffness was divided into four groups according to the elasticity quartiles. To facilitate operation, the stiffness (YM) was rounded to the closest integer to generate the score. Binary logistic regression was employed to evaluate the contribution of the parameters to the ischemic events. We performed the univariable logistic regression analysis and included variables with univariable P<0.05 in the multivariable logistic regression analysis. Based on the corresponding β coefficient on multivariable logistic regression, we derived the USR score. The sum of the risk parameter scores was added as the overall score. The discrimination ability of the models to identify symptomatic plaque was assessed using the C statistic. For internal validation, we used 5-fold cross-validation with 20 iterations in the model derivation datasets.
Statistical analysis and model construction were performed with SPSS 22.0 (SPSS Inc., Chicago, IL, USA) software and R 3.6.3 (https://cran.r-project.org/) (SPSS Inc., Chicago, IL, USA) software. Glm, rms, caret, pROC, and PredictABEL packages were used for R analysis. Values of P<0.05 were considered to be statistically significant.
Results
Demographic characteristics
Of 131 patients recruited, 33 patients were excluded according to exclusion criteria, 98 patients were available for the final analysis: 50 patients were divided into the symptomatic group, and the remaining 48 patients were divided into the asymptomatic group (Figure 1).
The demographic characteristics of the available patients in the two groups (symptomatic and asymptomatic groups) are shown in Table 1. Briefly, the mean age of the included patients was 65.6 years, and 76.5% were men. The two groups did not differ in age, sex, smoking and drinking history, diabetes mellitus, and hypertension. Usage of medications also did not show significant differences between the two groups.
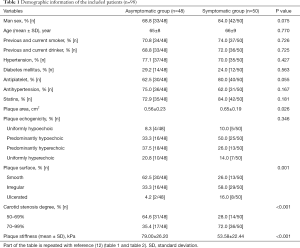
Full table
Plaque characteristics
Overall, 54.1% had severe stenosis (70–99%), and 45.9% had moderate stenosis (50–69%) on carotid ultrasound. The smooth plaque surface was in 43 of the 98 (43.9%) plaques, irregular in 45 (45.9%) plaques, and ulcerated in 10 (10.2%) plaques. The plaque elasticity ranged from 23.2 to 147.8 kPa, and the symptomatic group (53.58±22.44 kPa) were softer than the asymptomatic group (79.00±26.20 kPa) (P<0.001) (Figure 2). Interobserver agreement for the assessment of plaque elasticity using SWE was favorable, with an ICC of 0.897 (95% CI, 0.815–0.944) between the two vascular sonographers.

Variables associated with ischemic events
Among the factors with univariable P<0.05, plaque area, plaque surface, plaque stiffness and carotid stenosis degree were included in the multivariable analysis after excluding factors showing significant multicollinearity. Multivariable logistic regression analysis revealed that plaque surface (OR 3.39, 95% CI, 1.43–8.02, P=0.006), plaque stiffness (OR 0.96, 95% CI, 0.94–0.98, P=0.001) and carotid stenosis degree (OR 3.70, 95% CI, 1.37–10.01, P=0.01) were independent risk parameters of ischemic events. Plaque area was not associated with ischemic events in the multivariable analysis. After adjusting for age and sex, the ORs for plaque surface, plaque stiffness and carotid stenosis degree were 3.21 (95% CI, 1.35–7.67, P=0.009), 0.96 (95% CI, 0.93–0.98, P<0.001), and 3.46 (95% CI, 1.25–9.60, P=0.017) respectively.
USR score scale
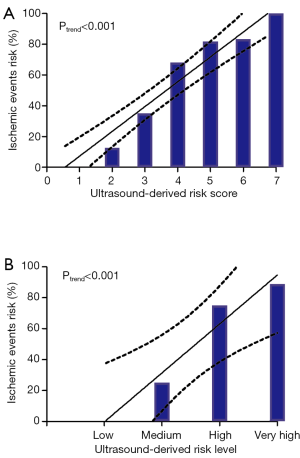
USR scores were assigned based on the observed β coefficient, and a USR scoring system was constructed (Table 2). The USR score scale (range, 0–7) incorporated three items: plaque surface (smooth, 0 points; irregular, 1 point; ulcer, 2 points), plaque stiffness (≥80 kPa, 0 points; 60–79 kPa, 1 point; 40–59 kPa, 2 points; <40 kPa, 3 points) and carotid stenosis (<50%, 0 points; 50–69%, 1 point; ≥70%, 2 points). Fifty-three (54.1%) patients had a score of 4 or more, and 3 patients (3%) had a score of 7. The risk of ischemic events increased with increasing USR score (P<0.001 for trend; Table 3 and Figure 3A). None of the patients with a score of 0 or 1 had ischemic events, whereas all 3 patients with a score of 7 had ischemic events. When the USR score was classified as low [0, 1], medium [2, 3], high [4, 5] and very high [6, 7] categories, a linear increase in ischemic event risk was observed (P<0.001 for trend; Table 4; Figure 3B). The ischemic event risk was 0% (0/9 patients) in the low category, 25.0% (9/36 patients) in the medium category, 75.0% (33/44 patients) in the high category, and 88.9% (8/9 patients) in the very high USR category.

Full table

Full table

Full table
Diagnostic performance of the USR score
In the threshold analysis, a score of more than 3 had 48% specificity but 96% sensitivity for discrimination of ischemic events. A score of more than 4 had 75% specificity and 82% sensitivity for ischemic events. On multivariable logistic regression analysis, after adjusting for age and sex, a 1-point increase in USR score was associated with ischemic event risk (adjusted OR 3.3, 95% CI, 2.03–5.26, P<0.001).
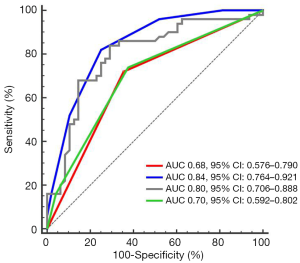
In the ROC analysis, the C statistic (0.84, 95% CI, 0.76–0.92) of the USR score for discriminating ischemic events was improved compared to plaque surface morphology (C statistic 0.70, 95% CI, 0.59–0.80), carotid stenosis degree (C statistic 0.68, 95% CI, 0.58–0.79), and plaque stiffness (C statistic 0.80, 95% CI, 0.71–0.89) (Figure 4). The 5-fold cross-validation yielded a C statistic of 0.82 (95% CI, 0.80–0.84).
USR score in moderate carotid stenosis
To investigate the association between the USR score and ischemic events in patients where additional information on lumen narrowing might be most helpful for clinical management relating to carotid revascularization, we further analyzed the data after excluding participants with severe lumen narrowing. Carotid stenosis was scored as zero or one, and the USR score ranged from 0–5. There were 45 patients with moderate carotid stenosis, with 14 ischemic events. In the logistic regression analysis, the crude OR of ischemic events in patients with moderate stenosis per 1-point increase in USR score was 8.41 (95% CI, 2.29–30.90, P=0.001). The C statistic of plaque surface morphology and plaque stiffness and USR score for discriminating ischemic events in patients with moderate stenosis were 0.67 (95% CI, 0.50–0.84), 0.81 (95% CI, 0.66–0.97), and 0.88 (95% CI, 0.78–0.98), respectively.
Discussion
We constructed a simple noninvasive risk score scale comprised of plaque surface morphology, plaque elasticity and carotid lumen narrowing to assess the risk of ischemic events in patients with proximal carotid stenosis. Our research provides several highlights. First, we reveal for the first time that the incorporation of plaque surface morphology, plaque elasticity and carotid stenosis in a single assessment can be used to identify patients at high risk of ischemic events. The probability of ischemic events increased gradually with increasing USR scores. No patients in the low USR categories had ischemic events, whereas all patients with a USR score of 7 had ischemic events.
Second, the relationship of the USR score with ischemic events was confirmed in the internal validation sample. In the derivation data, each 1-point increase in the USR score was associated with a 3-fold increase in the risk of ischemic events after adjusting for age and sex. A USR score of more than 3 had 96% sensitivity and 79% accuracy for the identification of ischemic events. In contrast, the C statistic for the discrimination of ischemic events was increased to 0.84 compared with plaque surface morphology (C statistic 0.70), carotid stenosis degree (C statistic 0.68) and plaque stiffness alone (C statistic 0.80), suggesting that the USR score had better discrimination ability for ischemic events, while SWE might be useful to guide clinical decisions.
Finally, the USR score has a desirable performance in analyses restricted to patients with moderate carotid stenosis, and the identification of patients at the highest risk of ischemic events is most likely to be clinically beneficial when considering selection for carotid revascularization.
Intraplaque hemorrhage, thin fibrous cap, large lipid core, neovascularization, and inflammation are all risk biomarkers of vulnerable plaque (20). Multiparameter ultrasound could help stroke risk stratification and identify patients who might require carotid intervention and who might not.
Irregular plaque surface, especially the presence of ulceration, indicates unstable plaques. Plaque ulceration was associated with intraplaque hemorrhage, a large lipid core, and decreased stability (21). Homburg et al. (22) showed that plaque ulceration is associated with non-lacunar ischemic stroke, independent of the severity of lumen stenosis (adjusted OR, 2.70; 95% CI, 1.43–5.09).
SWE is a novel noninvasive technique developed to quantify tissue elasticity and to differentiate lesion nature (23). Ramnarine et al. (24) reported that there were significant differences in plaque YM between symptomatic patients and asymptomatic patients (62 vs. 88 kPa; P=0.01). In our study, symptomatic plaques had a significantly lower mean YM than the asymptomatic plaques (53.6 vs. 79 kPa; P<0.001). The presence of intraplaque hemorrhage and lipid core at histology was associated with a significantly lower YM (25). A recent study showed a high concordance of 81.4% between SWE and computed tomography angiography (CTA) in evaluating the correlation between “soft plaques” and the presence of a lipid core (26). However, CTA is a complex and minimally invasive imaging modality that cannot be used as a tool to screen plaque vulnerability in asymptomatic patients.
In clinical practice, there is an overlap in YM values between stable and unstable plaques; therefore, it is insufficient to identify plaque characteristics that only depend on SWE. To our knowledge, no studies have systematically investigated the clinical utility of plaque surface morphology and plaque elasticity combined with carotid stenosis to identify patients at high risk of stroke.
Some limitations of the study should be acknowledged. First, there was no ultrasound imaging of patients with carotid lesions prior to ischemic event occurrence. Second, in some subgroup analyses, as relatively few patients had scores of 1 or 7, the confidence interval is wide. Larger sample studies are needed to improve the performance of risk estimation of the score. Third, SWE assessment is affected by the pulse motion of the carotid arteries, and the measurement of plaque YM may lead to errors. Fourth, all included patients had more than 50% proximal carotid stenosis; thus, the possible culprit plaques that caused stroke independent of lumen stenosis may have been missed in these patients. Fifth, the exclusion of plaques with poor image quality may lead to a selection bias. In addition, unmeasured confounding factors by variables not available in our study may inevitable. Finally, we validated the USR score scale only by internal samples, and a large-scale study of external validation is also needed in the future.
Conclusions
The USR score is a grading scale that assesses the risk of ischemic cerebrovascular events in patients with proximal carotid stenosis with preferable discrimination performance. A selection algorithm that combines information about plaque surface morphology, plaque elasticity and carotid stenosis might be more useful for patients who had less benefit from revascularization when clinical decisions only depend on lumen narrowing. Large-scale external validation with long-term follow-up in other studies will be needed to prove the predictive value.
Acknowledgments
Funding: This research was supported by the National Natural Science Foundation of China (ID 8173000716).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-8205
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-8205
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-8205
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-8205). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Review Board approval was obtained to perform this study (IRB No. KY2019-113-01). Informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- . GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study2016. Lancet Neurol 2019;18:439-58. [Crossref] [PubMed]
- Ooi YC, Gonzalez NR. Management of extracranial carotid artery disease. Cardiol Clin 2015;33:1-35. [Crossref] [PubMed]
- Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischaemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160-236. [Crossref] [PubMed]
- Abbott AL, Paraskevas KI, Kakkos SK, et al. Systematic Review of Guidelines for the Management of Asymptomatic and Symptomatic Carotid Stenosis. Stroke 2015;46:3288-301. [Crossref] [PubMed]
- Camps-Renom P, Prats-Sánchez L, Casoni F, et al. Plaque neovascularization detected with contrast-enhanced ultrasound predicts ischaemic stroke recurrence in patients with carotid atherosclerosis. Eur J Neurol 2020;27:809-16. [Crossref] [PubMed]
- Yamada K, Kawasaki M, Yoshimura S, et al. High-Intensity Signal in Carotid Plaque on Routine 3D-TOF-MRA Is a Risk Factor of ischaemic Stroke. Cerebrovasc Dis 2016;41:13-8. [Crossref] [PubMed]
- Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Eur Heart J 2018;39:763-816. [Crossref] [PubMed]
- Scoutt LM, Gunabushanam G. Carotid Ultrasound. Radiol Clin North Am 2019;57:501-18. [Crossref] [PubMed]
- Makris GC, Lavida A, Griffin M, et al. Three-dimensional ultrasound imaging for the evaluation of carotid atherosclerosis. Atherosclerosis 2011;219:377-83. [Crossref] [PubMed]
- Amamoto T, Sakata N, Ogata T, et al. Intra-plaque vessels on contrast-enhanced ultrasound sonography predict carotid plaque histology. Cerebrovasc Dis 2018;46:265-9. [Crossref] [PubMed]
- Lou Z, Yang J, Tang L, et al. Shear wave elastography imaging for the features of symptomatic carotid plaques: a feasibility study. J Ultrasound Med 2017;36:1213-23. [Crossref] [PubMed]
- Li Y, Zheng S, Zhang J, et al. Multimodal ultrasound parameters aided carotid plaque risk stratification in patients with asymptomatic carotid stenosis. Acta Radiol 2021; Epub ahead of print. [Crossref] [PubMed]
- Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35-41. [Crossref] [PubMed]
- Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: grayscale and Doppler ultrasound diagnosis-Society of Radiologists in Ultrasound consensus conference. Ultrasound Q 2003;19:190-8. [Crossref] [PubMed]
- Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008;21:93-111; quiz 189-90. [Crossref] [PubMed]
- Nicolaides AN, Kakkos SK, Kyriacou E, et al. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg 2010;52:1486-1496.e1-5.
- Elkind MS, Cheng J, Boden-Albala B, et al. Northern Manhattan Stroke Study. Elevated white blood cell count and carotid plaque thickness:the northern manhattan stroke study. Stroke 2001;32:842-9. [Crossref] [PubMed]
- Arnold JA, Modaresi KB, Thomas N, et al. Carotid plaque characterization by duplex scanning: observer error may undermine current clinical trials. Stroke 1999;30:61-5. [Crossref] [PubMed]
- de Bray JM, Baud JM, Dauzat M. Consensus Concerning the Morphology and the Risk of Carotid Plaques. Cerebrovasc Dis 1997;7:289-96. [Crossref]
- Saba L, Saam T, Jäger HR, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol 2019;18:559-72. [Crossref] [PubMed]
- Lovett JK, Gallagher PJ, Hands LJ, et al. Histological correlates of carotid plaque surface morphology on lumen contrast imaging. Circulation 2004;110:2190-7. [Crossref] [PubMed]
- Homburg PJ, Rozie S, van Gils MJ, et al. Atherosclerotic plaque ulceration in the symptomatic internal carotid artery is associated with nonlacunar ischaemic stroke. Stroke 2010;41:1151-6. [Crossref] [PubMed]
- Bamber J, Cosgrove D, Dietrich CF, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med 2013;34:169-84. [Crossref] [PubMed]
- Ramnarine KV, Garrard JW, Kanber B, et al. Shear wave elastography imaging of carotid plaques: feasible, reproducible and of clinical potential. Cardiovasc Ultrasound 2014;12:49. [Crossref] [PubMed]
- Garrard JW, Ummur P, Nduwayo S, et al. Shear Wave Elastography May Be Superior to Greyscale Median for the Identification of Carotid Plaque Vulnerability: A Comparison with Histology. Ultraschall Med 2015;36:386-90. [Crossref] [PubMed]
- Di Leo N, Venturini L, de Soccio V, et al. Multiparametric ultrasound evaluation with CEUS and shear wave elastography for carotid plaque risk stratification. J Ultrasound 2018;21:293-300. [Crossref] [PubMed]



