
Two-stage S7 sleeve resection of the right lower lobe and S1+2 and S3 segmentectomy of the left upper lobe: a case report
Introduction
For a patient with the small and peripheral lung cancers and/or GGOs in screening programs which might be diagnosed as stage I non-small cell lung cancer (NSCLC), surgical resection remains the first choice on the condition that the patient is functionally operable. In clinical practice, multiple primary pulmonary nodules often co-exist with benign and malignant nodules. The most common benign lung tumors are pulmonary hamartomas, which have an incidence of 0.025% and 0.32%. Although some studies have reported a higher incidence of 10%, endobronchial hamartomas (EHs) account for only 1.4% of pulmonary hamartomas (1). Low dose Computed tomography (LDCT) can detect small nodules with ground-glass opacities (GGOs) in the lung more effectively than conventional dose CT. With the application of high-resolution imaging systems worldwide, a large number of multiple lung nodules have been confirmed as synchronous multiple primary lung cancers (SMPLCs). Although the incidence rate of SMPLC varies from 0.2% to 8%, the 5-year overall survival rate of SMPLC can reach 82% after surgery (2). With an increased number of patients presenting with SMPLCs, the need for sub-lobar lung resection procedures, such as wedge resection and segmentectomy, instead of lobectomy, is increasing. Some benign lung tumors that block the bronchial orifice may also require surgical intervention. Based on the characteristics, location, and size of nodules, and the patient’s status, therapy should be individualized as much as possible to reserve the function of lungs and prolong overall survival. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/atm-21-1570).
Case presentation
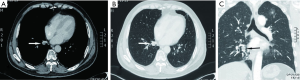
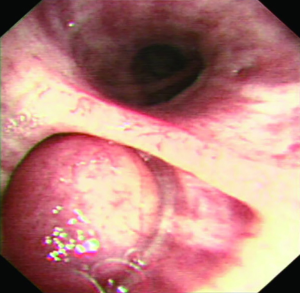
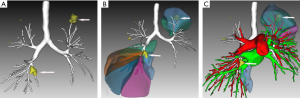
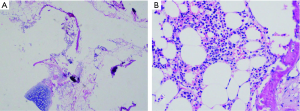
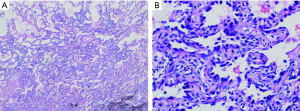
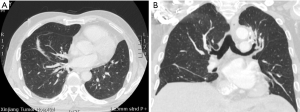
A 56-year-oldman, who was a pack-a-day smoker, presented with a complaint of persistent cough for 10 days. He denied fever, chill, chest pain, or weight loss and showed no abnormal physical findings, including abnormal breathing. The patient had a family history of lung cancer, and one of his direct relatives had died of lung cancer. Plate fixation was performed because his left upper limb (LUL) was fractured 15 years prior. The patient was evaluated for various etiologies of cough to obtain the correct diagnosis. No obvious abnormalities were detected by way of hemogram, blood biochemistry, or blood gas analysis. Pulmonary function tests did not show any obstruction. Vital capacity and forced expiratory volume in 1 s (FEV1) were 3.82 and 2.96 L, respectively. Chest CT scan showed an endobronchial nodule in the medial basal bronchus (B7) in the right lower lobe (RLL) (Figure 1A,B,C). Mixed GGOs (mGGOs) were detected in the LUL (16×18 mm) (Figure 2A,B). The CT did not suggest obstructive atelectasis or pneumonia in the RLL. CT was followed by bronchoscopy, which revealed a smooth hyperemic mass occluding the orifice of the B7 in the RLL (Figure 3). The standard approach of removing the tumor with biopsy forceps through thin flexible bronchoscopy was rejected due to the size and location of the tumor. Pathological examinations of specimens obtained by bronchoscopy did not reveal a definite diagnosis. Preoperative 3D reconstruction revealed the location and size of lesions of the B7 in the RLL and mGGOs of S1+2 and S3 in the LUL, which may provide guidance for surgical treatment (Figure 4A,B,C). Sleeve segmentectomy was then performed on the medial basal segment (S7) in the RLL via muscle-sparing incision and a thoracoscope. The diagnosis of hamartoma of lesions located in the B7 following analysis of the intraoperative frozen section. There were no tumors in the residual edge of the B7. The patient was discharged from hospital after 14 days following an uneventful postoperative period. The final diagnosis confirmed hamartoma in the B7 via postoperative histopathology (Figure 5A,B). 10 days later, postoperative bronchoscopy showed there was no stenosis and occlusion in anastomotic stoma(Figure 6). After 3 months, the patient underwent video-assisted S1+2 and S3 segmentectomy of the LUL. Intraoperative pathology showed invasive adenocarcinoma as the postoperative histopathology (Figure 7A,B). The patient recovered well and was discharged after 17 days. His symptoms improved significantly after surgery.
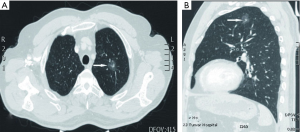

At the 3-month follow-up, CT and bronchoscopy indicated no stenosis of anastomosis in the RLL (Figure 2B), and there was no evidence of disease recurrence (Figure 8A,B). The patient underwent a lung function test and the results were compared with the preoperative lung function test. Forced vital capacity(FVC), forced expiratory in first second (FEV1), maximal ventilatory volume (MVV), and diffusion capacity of the lung for carbon monoxide (DLCO) did not significantly decrease and the patient was in good condition at follow-up. At present, the patient is still being followed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
Endobronchial mass can vary depending on the location and size of the tumor. Differential diagnoses for such lesions are bronchogenic carcinoid, non-small or small cell carcinoma, arteriovenous malformation, lymphoma, and metastases in the lung (3). The patient may be asymptomatic, especially in early stages, but may develop a persistent cough, as was the case with our patient. Airway obstruction can manifest as dyspnea, atelectasis, and recurrent pneumonias. EHs usually have clear boundaries and are small, with an average size of 1.5 cm. However, some have been reported to grow to about 6 cm (4,5). Due to extensive growth, EHs is prone to an atelectasis of the lung, resulting in respiratory insufficiency. However, in 30% of cases, CT findings clearly indicate the absence of a benign lesion (calcifications, fat) (6); only 15% of EHs are diagnosed prior to surgery (7). Therefore, bronchoscopy or open biopsy may be required for a definitive diagnosis.
In the present case, the diagnosis was made via biopsy of intraoperative rapid frozen sections. Treatment should be individualized on the basis of the site, size, and extent of the tumor, as well as the patient’s comorbidities. In the present case, the bronchoscopic resection was rejected after considering the size and location of the tumor, together with the risk of the atelectasis and pneumonia due to recent bleeding, as well as the impact on the treatment of possible malignant tumors in the RLL.
Surgery is still the recommended treatment for an endobronchial tumor that is difficult to be diagnosed or removed by bronchoscopic approach (8), especially when the tumor causes irreversible lung damage and recurrent infections at the distal site of obstruction. By avoiding lobectomy, sleeve segmentectomy becomes possible in this case of compromised pulmonary function, while also benefiting patients with mGGO who need further treatment. The reasons for selecting sleeve segmentectomy following intraoperative diagnosis in the present study were as follows: (I) to preserve as much lung parenchyma as possible if further surgeries for early-stage carcinoma in other lobes were required; (II) the patient had no previous history of respiratory infections and no adjacent trachea injuries, and there were no signs of inflammation and edema in the specimen during the operation; (III) although there was a very low probability of EH recurrence and malignant degeneration in hamartomas, the recurrence of tumor growth with narrowing of the RLL bronchus may cause atelectasis and an ongoing cough; (IV) post-obstructive pneumonia cannot be treated with antibiotics alone because of poor drainage of pulmonary parenchyma related to edema of anastomotic stoma, and can lead to rehospitalization, increased medical costs, and adverse outcomes; and (V) the purpose of reconstruction of the anatomical structure of the trachea or bronchus is to decrease the impact of surgery on lung function These reasons indicate that surgical treatment with sleeve segmentectomy of S7 in the RLL was suitable in our patient.
Sleeve resection was initially offered as an alternative to patients with marginal pulmonary function. The key point is the tumor extension from segmental bronchi to the lobar or main bronchial orifice. Currently, it is increasingly applied to patients with more normal functions, and preserves pulmonary parenchyma (9). However, the performance of S7 sleeve segmentectomy of the RLL is rare. Liao et al. reported a 42-year-old man was discovered to have an endobronchial tumor, and performed the left lower lobe sleeve resection (10). Postoperative histopathological findings confirmed a benign schwannoma. Common complications include sputum retention and secondary atelectasis, bronchovascular and bronchopulmonary fistula, and anastomotic failure. The main concern is stenosis of the anastomosis, and the major technical striking point is the caliber discrepancy between proximal and distal bronchi (11). To avoid postoperative complications and ensure a good functional result, anastomosis between bronchi must be performed precisely. Several techniques, such as telescoped bronchial anastomosis, enlarging the circumference of the small bronchus with an oblique section, or reducing the caliber of the larger bronchus by crimping its posterior membrane, have been described to resolve this problem (12). In our experience, a series of preventive measures was taken for anastomotic stenosis. First, after adjusting the proximal bronchial stump by bending and then suturing the non-cartilaginous posterior part of the bronchial wall, anastomosis between the basal bronchus was performed with continuous and repeated suture using a 4-0 polydioxanone suture. Second, to prevent anastomotic stenosis of organs of the middle lobar bronchus, and anterior-, lateral-, and posterior-basal bronchi, preoperative 3D reconstruction, owing to its capacity to fully identify the abovementioned structures, was performed to ensure that the distance from those to the B7 was >3 mm. Third, at the commencement of anastomosis, a traction line is sutured on the cartilage side at the junction of the segmental bronchial membrane. This is more conductive to the smooth anastomosis and prevention of twining of suture lines. Furthermore, it is necessary to pay attention to the anastomosis of the end-to-end anastomosis, as well as the anastomosis of the segmental bronchial membrane to membrane and cartilage to cartilage. Finally, during the operation, 4-0 sliding sutures are used to continuously suture the end of the anastomosis, with consideration of the spacing. The anastomosis of the cartilage is completed first, followed by the tensionless anastomosis of the membrane.
Bronchoplastic procedures for benign and low-grade malignant tumors of the airway and benign stenosis allow preservation of maximum amount of pulmonary parenchyma. Benign and low-grade malignancies require only minimally clear margins for cure and are ideally suited to bronchoplastic resections. Resection was considered complete and efficient when the margins were free of disease and achieve considerable long-term prognosis. The results of the present study support the view that, with high-tech methods, such as 3D reconstruction, and proper patient selection, complications related to S7 sleeve segmentectomy in the RLL can be avoided. This case provides a novel approach for the treatment of patients with multiple primary early-stage lung cancer and benign endobronchial tumors. Furthermore, it is worth emphasizing that regular follow-up and CT of ≤1.5 mm-thick sections are also important for long-term results.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/atm-21-1570
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-21-1570). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El-Kersh K, Perez RL, Gauhar U A. 63-year-old man with a chronic cough and an endobronchial lesion. Diagnosis: Endobronchial hamartoma. Chest 2014;145:919-22. [Crossref] [PubMed]
- Zhang Z, Gao S, Mao Y, et al. Surgical Outcomes of Synchronous Multiple Primary Non-Small Cell Lung Cancers. Sci Rep 2016;6:23252. [Crossref] [PubMed]
- van den Bosch JM, Wagenaar SS, Corrin B, et al. Mesenchymoma of the lung (so called hamartoma): a review of 154 parenchymal and endobronchial cases. Thorax 1987;42:790-3. [Crossref] [PubMed]
- Sarioglu N, Susur A, Goksel T, et al. An unexpected cause of hemoptysis: endobronchial lipomatous hamartoma. Med Arch 2014;68:65-6. [Crossref] [PubMed]
- Zehani-Kassar A, Ayadi-Kaddour A, Marghli A, et al. Clinical characteristics of resected bronchial hamartoma. Study of seven cases. Rev Mal Respir 2011;28:647-53. [Crossref] [PubMed]
- Radosavljevic V, Gardijan V, Brajkovic M, et al. Lung hamartoma--diagnosis and treatment. Med Arch 2012;66:281-2. [Crossref] [PubMed]
- Mertoğlu A, Tellioğlu E, Yücel N. Multiple endobronchial hamartoma. Clin Respir J 2017;11:263-6. [Crossref] [PubMed]
- Karabulut N, Bir F, Yuncu G, et al. Endobronchial lipomatous hamartoma: an unusual cause of bronchial obstruction (2007: 7b). Eur Radiol 2007;17:2687-90. [Crossref] [PubMed]
- Wright CD. Sleeve lobectomy in lung cancer. Semin Thorac Cardiovasc Surg 2006;18:92-5. [Crossref] [PubMed]
- Liao H, Song W, Chen N, et al. Left lower lobe sleeve resection for endobronchial schwannoma. Ann Transl Med 2019;7:50. [Crossref] [PubMed]
- Ohata K, Zhang J, Ito S, et al. Right lower lobe sleeve resection: bronchial flap to correct caliber disparity. Ann Thorac Surg 2013;95:1107-8. [Crossref] [PubMed]
- Boudaya MS, Abid W, Mlika M. Sleeve Right Lower Lobectomy: a Rarely Performed Extended Resection. Indian J Surg 2016;78:74-6. [Crossref] [PubMed]
(English Language Editor: R. Scott)


