Recombinant human bone morphogenetic protein-4 enhances tendon-to-bone attachment healing in a murine model of rotator cuff tear
Introduction
Tendon-to-bone attachment (TBA) transfers the force from muscles to the bone during bodily movement, and the involved structure comprises 4 distinct yet continuous zones: tendon, nonmineralized fibrocartilage, mineralized fibrocartilage, and bone (1-4). Injuries of TBA are commonly seen in the clinic; an example is rotator cuff tear. Unfortunately, regeneration of the special tissues, particularly the native fibrocartilaginous enthesis, is not possible, partly due to the two materials' vastly different mechanical properties. Surgical repair of the structure entails attaching the tendon to the bone via anchors, and the tendon is mended to the bone via a fibrovascular scar tissue, which has inferior biomechanical properties. It has been reported that the rate of re-rupture following rotator cuff repair is 10–28% from minor to large tears (5). Numerous therapies have attempted to regenerate the native fibrocartilaginous enthesis, such as physical therapy, stem cells, biomechanical scaffolds, and biologic agents, but only limited success has been achieved to date (6,7).
A better understanding of the fundamental mechanism of TBA healing may promote new approaches to facilitate healing. Bone morphogenetic protein-4 (BMP-4), a member of bone morphogenetic proteins, is well known for its acceleration of inducing bone formation. However, an increasing volume of research has demonstrated the potential for BMP-4 to promote chondrogenesis (8-10). Recombinant human BMP-4 (rhBMP-4) was found to stimulate chondrogenesis in limb buds by inducing cartilage matrix production, which was important to maintain the cartilage phenotype. The rhBMP-4 could successfully promote the initial differentiation of mesenchymal stem cells (MSCs) in vitro to become chondroprogenitor lineage and induce differentiation into mature chondrocytes (11). Muscle derived stem cells (MDSCs) that expressed BMP-4 was able to promote cartilage regeneration in a murine articular cartilage defect model, without degradation or ossification for up to 6 months postoperatively (8). When MDSCs expressing BMP-4 and sFlt1 with platelet rich plasma (PRP) were delivered to assist articular cartilage repair, collagen synthesis was promoted, chondrocyte apoptosis was suppressed, and finally, the repair process was enhanced (12).
Moreover, when MSCs were co-treated with transforming growth factor beta (TGF-β) and BMP-4, chondrogenesis was further improved via shortening the duration of SOX 9 protein decline (13). Therefore, the role of BMP-4 in chondrogenesis was viewed as significant, and it might be a promising therapy for cartilage defects (11). However, it is still unknown whether BMP-4 can enhance the formation of fibrocartilaginous enthesis in the attachment between the rotator cuff tendon and humerus after its surgical repair. Since chondrogenesis also plays a crucial role in TBA healing, we hypothesized that BMP-4 might be involved in TBA healing and regulate enthesis regeneration.
The goal of this study was to investigate the role of BMP-4 in augmenting TBA healing. In this study, the effect of rhBMP-4 and noggin (an inhibitor of all BMP activities) were detected in a murine rotator cuff repair model, concerning the micro-computed tomography (micro-CT), histomorphology, and biomechanical properties.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-6761).
Methods
Study design
All procedures complied with the Animals (Scientific Procedures) Act 1986 and received university ethical approval of Xiangya Hospital, Central South University (No. 2019030490). A total of 120 mature (12 weeks, 22±3 g) male C57 black (BL)/6 mice were used in this study, and a rotator cuff repair model was established in the left forelimb, following the method of Dr. Scott Rodeo (14). The mice were randomly allocated to 1 of 3 groups: control (n=40), rhBMP-4 (n=40), and noggin (n=40), and were then intervened with fibrin sealant, fibrin sealant containing rhBMP-4 (Bio-tool, Beijing, China), and fibrin sealant containing noggin (Huabio, Hangzhou, China), respectively. Previous studies have shown that fibrin sealant does not promote rotator cuff healing (15); therefore, the natural healing group was canceled in this study. Animals were housed in specific-pathogen free (SPF) animal houses (5 companions per cage) on a standard diet and allowed unrestrained cage activity after surgery. At postoperative 2 and 4 weeks, mice were sacrificed, and bilateral supraspinatus tendon-humerus (SSTH) complexes were harvested for subsequent investigations (Figure 1).
Fibrin sealants preparation and in vitro drug sustained-release test
Fibrinogen solution (Sigma-Aldrich, St. Louis, MO, USA) and thrombin solution (Sigma-Aldrich) were prepared following the manufacturer’s manual. Then, RhBMP-4 and noggin were added to the thrombin solution, respectively. A total of 10 μL fibrinogen solution and 2 μL thrombin solution [containing 0.5 μg rhBMP-4 or 0.2 ng noggin, at the dosage used in prior studies (16,17)] were utilized to make each fibrin sealant, with a 0.22 μm syringe filter (Millex, Sigma, St. Louis, MO, USA). Similarly, fibrin sealant was also prepared in the control group, but it contained no active ingredients.
We measured the BMP-4 and noggin release kinetics from the fibrin sealant, which was comprised of 250 μL fibrinogen solution and 50 μL thrombin solution, and 12.5 μg rhBMP-4 or 5 ng noggin. The gels were incubated in 0.5 mL phosphate buffered saline (PBS) with 10% fetal bovine serum (FBS) at 37 °C. The PBS solution was replaced and collected 5 times over the first 24 hours, and was then changed and collected daily. Enzyme-linked immunosorbent assay (ELISA) for BMP-4 and noggin were performed on the washed solutions, respectively. The release experiment was carried out 3 times, and cumulative percentages are presented in the results.
Surgical technique
Briefly, mice were placed in a right lateral decubitus position after being anesthetized with an intraperitoneal injection of 0.3% pentobarbital sodium. An incision was made on the left shoulder, and the deltoid muscle was revealed, which was minimally dissected to expose the supraspinatus tendon (SST). Using a customized retractor to elevate the acromion, the SST was isolated. Then, the SST was sutured with a 6-0 prolene with double needles (Ethicon, Somerville, NJ, USA) in an “8” figure fashion, and sharply detached from its footprint on the humeral head. Any soft tissues and fibrocartilaginous tissues remaining on the bone were gently removed with a scalpel. A transosseous tunnel was then created with a 30-G needle in the humeral head under the footprint, and the two sutured limbs were crossed through the tunnel, one from lateral to the interior and the other from interior to lateral. After tightening the two sutured limbs, the SST was reattached to the footprint. Before tying the knot, fibrin sealant, fibrin sealant containing rhBMP-4, or fibrin sealant containing noggin was placed on the tendon-to-bone interface. Finally, the wounds were layered sutured, and buprenorphine (0.05 mg/kg) was administrated subcutaneously for postoperative analgesia. Two sports medicine doctors performed all animal surgeries under surgical microscopy.
Micro-computed tomography (CT) evaluation
Micro-CT (viva CT 80; Scanco Medical, Brüttisellen, Switzerland) was performed in this study to assess the formation of subchondral bone at the tendon attachment site. After fixing in 10% paraformaldehyde at 4 °C for 24 hours, each specimen was kept in a tube surrounded by formalin and scanned with a 10.4 μm voxel size, at 55 KVp, 0.36° rotation step (180° angular range), and a 400 ms exposure per view. Then, each specimen’s data were processed with a 3-dimensional Gaussian filter and a global threshold to identify the bone voxels from the surrounding saline in the bone marrow and soft tissues. After thresholding, the micro-parameters of osteogenesis at the TBA were calculated, including bone volume fraction (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), and trabecular spacing (Tb.Sp). The region of interest (ROI) was selected randomly under the footprint at the center of the 2 suture bone holes in the image. In the analysis, the ROI was defined as a cube with a length of 1 mm from the bone surface and extending distally to avoid the adjacent cortical bone’s involvement.
Histomorphometric analysis
After dissection, the fresh SSTH structures were fixed in 10% neutral buffered formalin for 48 hours. After 7 days of decalcification in 10% ethylenediaminetetraacetic acid (EDTA), the specimens were dehydrated in grade ethanol and embedded in paraffin. The paraffin blocks were sectioned with 5 µm thickness in the coronal plane through the SST and the greater tuberosity.
The sections were stained with hematoxylin and eosin (H&E, Solarbio Co. Ltd, Beijing, China) and toluidine blue-fast green (Sigma-Aldrich, St. Louis, MO, USA). The H&E stained sections were used for the histological description of TBA healing and semi-quantitative analysis for cell density around the tendon to the bone interface (15). Toluidine blue O/fast green (TB) stained sections were used for semi-quantitative analysis of the fibrocartilage zone thickness using the ImageJ software (National Institutes of Health, Bethesda, MD, USA) (18). All sections were observed using transmitted light microscopy (Olympus CX31; Olympus Inc., Tokyo, Japan) under the same conditions. The histological images were semi-quantitatively analyzed by two blinded observers (with qualifications: MD or PhD).
Immunofluorescence analysis
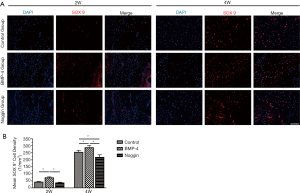
Immunofluorescence was used to detect SOX 9 expression cells in the tendon’s interface zone to the bone. After fixation, decalcification, dehydration, and embedding in an optimal cutting temperature compound, the SSTHC specimens were longitudinally sectioned with 10 µm thickness. For immunofluorescence staining, the sections were washed with PBS, permeabilized with 0.1% TritonX-100 (Sigma-Aldrich, St. Louis, MO, USA), and blocked with 5% bovine serum albumin (BSA) (Sigma-Aldrich). Then, a primary antibody against mouse SOX 9 (ab76997, Abcam, Cambridge, UK) was applied at 4 °C ~10 hours, subsequently incubated with anti-rabbit secondary antibody (ab150116, Abcam, Cambridge, UK) at room temperature for 1 hour, and finally incubated with 4’,-diamidino-2-phenylindole (DAPI) (ab228549, Abcam). All images were observed and captured using a Zeiss Axio Imager (Zeiss, Solms, Germany). According to a previous similar study to quantitate the positive signals of SOX 9, a ROI was defined at the SST attachment site (19). Briefly, the ROI, 0.5 mm × 0.4 mm in size, was located in the fibrocartilage layer of the SST insertion near the humeral head’s articular surface. The SOX 9 positive cell areas were counted 3 times, and the mean number per square millimeter of each sample was reported. The two assessors (with qualifications: MD or PhD) were blinded to the groups during image analysis.
Biomechanical testing
For biomechanical testing, each SSTHC was carefully dissected from surrounding tissues under a surgical microscope, and stored at −80 °C. At the time of testing, the specimen was thawed at room temperature, and the supraspinatus muscle and all sutures were carefully removed. Before biomechanical testing, each specimen was wrapped in surgical gauze soaked with 0.9% saline solution water and placed in a specimen box, which was then put in a 37 °C water bath environment. The humerus was mounted, and the SST was fixed in custom grip with sandpaper and ethyl cyanoacrylate. A microcomputer-controlled electronic material testing system (WD-T, Shanghai Zhuoji Instrument Equipment Co., Ltd., Shanghai, China) was used to test TBA’s failure load. The SSTHC was placed in the testing system machine and subjected to uniaxial tension following the SST’s approximate anatomic position. After pre-tensioning 3 times under a preload of 0.0–0.5 N, the SSTHC was loaded to failure at a 1 mm/min rate, and the ultimate failure load was recorded. The stiffness was calculated from the curve of the load to deformation.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 6.0.1; San Diego, CA, USA). All quantitative data are presented as mean ± SD (standard deviation) and were analyzed statistically using a 2-way analysis of variance (ANOVA) with the Bonferroni post hoc test to detect differences among groups. The significance level was set at P<0.05.
Results
All animals tolerated the surgery, and there were not any perioperative complications. Either group showed no gross failures.
In vitro release kinetics
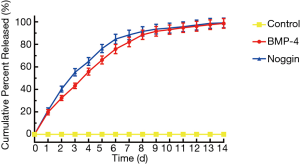
Sustained release of BMP-4 and noggin were achieved through the use of the fibrin gel system. In the first 24 hours, (19.6±2.4)% and (21.2±2.4)% of the BMP-4 and noggin were released into the PBS-FBS solution, respectively. At the end of 14 days, nearly (98.6±4.3)% and (99.1±4.3)% of the total BMP-4 and noggin were released into the solution, respectively (Figure 2).
Micro-CT evaluation
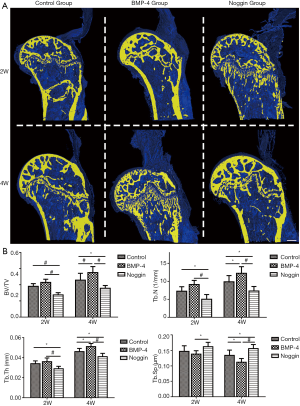
The three-dimensional morphologic of the SSTH was constructed, and the microstructural parameters of the subchondral bone at the attached interface were analyzed (Figure 3). The microstructure data showed that the BMP-4 group had significantly greater BV/TV (0.551±0.075), Tb.N (12.225±1.830 mm-1), and Tb.Th (0.051±0.0031 mm) at postoperative 4 weeks than the control group (BV/TV: 0.458±0.084, P<0.01; Tb.N: 9.864±1.730 mm-1, P<0.05; Tb.Th: 0.046±0.0032 mm, P<0.05), while there was no difference at 2 weeks for both (BV/TV: 0.430±0.039 vs. 0.381±0.033, P>0.05; Tb.N: 9.108±1.143 vs. 7.255±1.238 mm-1, P>0.05; Tb.Th: 0.036±0.0037 vs. 0.034±0.0028 mm, P>0.05). In the noggin group, the BV/TV (2 wk: 0.275±0.027; 4 wk: 0.357±0.034), Tb.N (2 wk: 5.013±1.308 mm-1; 4 wk: 7.310±1.253 mm-1),and Tb.Th (2 wk: 0.029±0.0024 mm; 4 wk: 0.041±0.0033 mm) were significantly lower than the control group (2 wk: P<0.01 for BV/TV; P<0.05 for Tb.N and Tb.Th; 4 wk: P<0.05 for BV/TV, Tb.N, and Tb.Th) and BMP-4 group (P<0.05 for BV/TV, Tb.N, and Tb.Th in both 2 and 4 wk) both at postoperative 2 and 4 weeks. Also, the Tb.Sp in the noggin group (2 wk: 0.162±0.014 mm; 4 wk: 0.156±0.013 mm) were higher than that in the control group (2 wk: 0.147±0.018 mm, P>0.05; 4 wk: 0.134±0.017 mm, P<0.05) at week-4 and BMP-4 group (2 wk: 0.138±0.011 mm, P<0.05; 4 wk: 0.112±0.012 mm, P<0.01) at week-2 and -4.
Histomorphometry
The H&E staining (Figure 4) showed that the SST was healed to the humeral head, and the enthesis was gradually regenerated with time. At postoperative 2 weeks, tissue connecting tendon and bone was fibrovascular and was poorly organized and highly cellular in all groups. In the BMP-4 and control group, there were some cartilage-like cells in the attachment zone, which was rare in the noggin specimens. The mean cell density in the TBA was lower in the BMP-4 groups (5,425±683 cells/mm2, P<0.05), and there was no significant difference between the noggin group (6,825±790 cells/mm2, P>0.05) and control group (6,539±720 cells/mm2) at week 2. At the postoperative 4 weeks, remolding was observed in the attachment, and an enthesis had reformed, characterized by prevalent fibrocartilage cells occurring between the tendon and bone. Mean cell density in the TBA was also lower in the BMP-4 group (4,339±544 cells/mm2, P<0.05) but higher in the noggin group (6,428±605 cells/mm2, P<0.05) when compared to the controls (5,410±582 cells/mm2) at week 4. Also, the mean cell density in the noggin group was significantly higher than that in the BMP-4 group at week-2 and -4 (P<0.01).
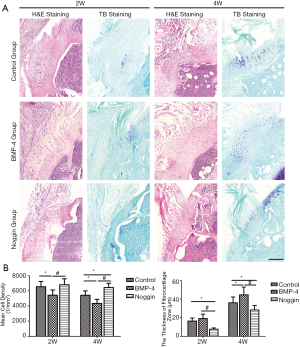
The TB staining (Figure 4) showed some TB deposited at the attachment site, which indicated the formation of fibrocartilage. At postoperative 2 weeks, the thickness of fibrocartilage zone in the noggin group (7.7±1.3 μm, P<0.05) was lower than the control group (16.4±3.3 μm), while there was no significant difference between the BMP-4group (19.1±4.7 μm, P>0.05) and the control group. At postoperative 4 weeks, the BMP-4 group (44.5±8.4 μm, P<0.05) had a thicker fibrocartilage zone, and the noggin group (28.3±4.8 μm, P<0.05) was thinner, compared with the control group (36.0±6.2 μm). Furthermore, the thickness of the fibrocartilage zone in the BMP-4 group was thicker than that in the noggin group at postoperative week-2 and -4 (P<0.01).
Immunofluorescence analysis
Immunofluorescence evaluation for SOX 9 (Figure 5) showed that SOX 9 positive cells were significantly more in the BMP-4 group (2 wk: 73.2±6.7 cells/mm2, P<0.05; 4 wk: 286.4±15.2 cells/mm2, P<0.05) than that in the control group (2 wk: 41.2±5.1 cells/mm2, P<0.05; 4 wk: 253.8±13.6 cells/mm2, P<0.05) at both postoperative 2 and 4 weeks. On the other hand, the number of SOX 9+ cells in the noggin group (217.5±18.6 cells/mm2, P<0.05) was less than that in the control group at week 4, and there was no significant difference between them at week 2 (35.6±5.4 vs. 41.2±5.1 cells/mm2, P>0.05). Also, compared with the BMP-4 group, numbers of SOX 9+ cells in the noggin group were lower at both weeks 2 and 4 (P<0.05 for both).
Biomechanical testing
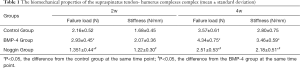
During the mechanical testing, the failure site in all groups was at the tendon-bone attachment. At postoperative 2 weeks, the BMP-4 group had a significantly higher failure load (P<0.05), and the noggin group had a significantly lower failure load (P<0.05) than the controls. There was no significant difference in stiffness in the BMP-4 group (P>0.05) and noggin group (P>0.05), when compared to the control group. At the postoperative 4 weeks, both the failure load and stiffness were higher in the BMP-4 group (P<0.05 for both) and lower in the noggin group (P<0.05 for both). Also, the failure load and stiffness in the BMP-4 group were significantly higher than those in the noggin group at postoperative weeks 2 and 4 (P<0.05 for both). All biomechanical testing data are shown in Table 1. According to the estimation, the power of testing of each outcome variable exceeded 95%.
Full table
Discussion
The current study showed that the application of rhBMP-4 fibrin sealant in the tendon bone repair interface promoted the formation of subchondral bone, fibrocartilage zone regeneration, and biomechanical property of the repaired enthesis in a murine model of rotator cuff repair. Combined with the lower cellular and higher SOX 9 expression, our data, therefore, demonstrated that BMP-4 seemed to accelerate TBA healing.
Chondrogenesis is a crucial factor contributing to the regeneration of attached enthesis in tendon to bone injuries, and the regenerated enthesis is closely related to the TBA’s biomechanical property. Histologically, this regenerated enthesis is featured in the organized fibrocartilage zone between bone and tendon (4). Chondrocyte density and fibrocartilage zone thickness were used as important indicators to evaluate the fibrocartilaginous enthesis. In the present study, we observed fibrocartilaginous entheses formed at postoperative 2 weeks in the murine rotator cuff repair models and found that fibrocartilage thickness was thicker at postoperative 4 weeks, with lower chondrocyte density. Besides, many studies have been performed to explore the effects of BMP-4 on chondrogenesis further, and BMP-4 has been shown to maintain chondrocyte phenotype by stimulating the synthesis of collagen type II, and suppressing the production of collagen type X (11,20). This was mostly associated with the interaction between Runx2 and BMP-activated Smad1. In vitro, BMP-4 facilitated the differentiation of MSCs to a chondroprogenitor lineage and induced differentiation towards mature chondrocytes (21). During MSC chondrogenesis, BMP-4 appeared to shorten the duration of SOX 9 protein decline (9,13). In the developing mouse mandibular process, BMP-4 could induce chondrogenesis, and it was positively regulated by SOX 9 (22). As the essential factor to restore the native enthesis, SOX 9 (23) was also observed in the tendon’s attachment site to the bone, and the number of SOX 9 positive cells was more than the controls. Therefore, BMP-4 might promote fibrocartilaginous enthesis regeneration in TBA healing by regulating the SOX 9 mediated chondrocyte differentiation.
Also, BMP-4 has a well-known positive effect on bone formation. Although the histological sections showed no obvious new bone formation at the TBA site, the microstructure of trabecular bone detected by micro-CT showed that the subchondral bone quality at the attachment site was better in the BMP-4 group compared to the control group or noggin group during the TBA healing. The BV/TV, Tb.N, and Tb.Th were higher in the BMP-4 group, and lower in the noggin group at postoperative weeks 4 and 8. Our previous studies have confirmed the crucial role of osteogenesis in the TBA healing (24,25); BMP-4 was able to facilitate new bone formation through endochondral ossification (26), which was found to be one initiating event during TBA healing (18,27). Meanwhile, BMP-4 facilitated mineralization by enhancing fibronectin synthesis and fibrillogenesis in osteoblasts (28). Interestingly, a gradient fibrocartilage zone in the mature TBA was evidenced as non-mineralized fibrocartilage and mineralized fibrocartilage, and mineralization of the attachment was vital to regenerate a mature rotator cuff enthesis (1,3). This study showed that BMP-4 improved the quality of the subchondral bone at the TBA and promoted the formation of the fibrochondrocyte. Thus, BMP-4 seemed to facilitate bone formation and mineralization and the formation and mineralization of the fibrocartilage layer during the TBA healing.
Noggin is a special molecule that prevents BMPs from interacting with their receptors (29). Noggin could inhibit BMPs inducing new bone formation via decreasing collagen synthesis (30). However, when noggin was knocked out, excess BMP activity was observed, and cartilage hyperplasia and joint formation failure resulted, while the noggin’s overexpression always led to the complete absence of skeletal elements (31). Noggin might play a regulating and balancing role on the effect of BMPs on chondrogenesis and osteogenesis. In rat anterior cruciate ligament (ACL) reconstructed models, noggin was shown to decrease new bone formation in the quality and increase fibrous scar tissue in the tendon-bone interface but did not affect the biomechanical properties (32). In the present study, noggin seemed to impair the SST healing to the humerus, reduce mineralization and fibrocartilage formation, and weaken ultimate failure load and stiffness at the postoperative 4 weeks.
The current study had several limitations. First, as BMP-4 was bound to extracellular matrix components, it was very difficult to detect the exact doses of BMP-4 secreted from fibrin sealants in vivo. The release kinetics of BMP-4 and noggin in vitro clearly showed that the fibrin sealant provided sustained release, but it could not represent the actual release kinetics in vivo. It is possible that the release of BMP-4 and noggin in vivo was slower or faster on account of the increased complexity of the injury site in vivo. Second, although the mouse is a great model for SST repair (14), mice are quadrupedal animals, and the mechanical stimulation after surgery is different from humans; thus, we cannot simply translate our findings into the rotator cuff tear repair in humans, further experiments are required to verify the effect of BMP-4 and noggin on TBA healing. Third, postoperative management was different from clinical practice. All animals were allowed free activity postoperatively in this study, while patients who have undergone surgical repair of rotator cuff injuries are asked to remain immobilized at an extended position using auxiliary equipment for several days. Finally, though all mice were operated on by the same operator and assistant who were trained well before the study, procedural variations were still inevitable, and the relatively small sample size may have led to statistical errors while concluding.
Conclusions
In summary, this study suggested that BMP-4 might play a vital role in the healing of TBA. The results showed rhBMP-4 had a positive effect on the regeneration of fibrocartilaginous enthesis and mineralization, while noggin inhibited this process.
The BMP-4 might be a potential therapy to augment TBA healing and lead to more rapid rehabilitation and reduce the risk of recurrent injury.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (81902220), the Natural Science Foundation of Hunan Province, China (2018JJ3814), and The Fundamental Research Funds for the Central Universities of Central South University (2017zzts238).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-6761
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-6761
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-6761). Dr. HC reports grants from the Fundamental Research Funds for the Central Universities of Central South University, during the conduct of the study. Dr. TZ reports grants from the National Natural Science Foundation of China, grants from the Natural Science Foundation of Hunan Province, China, during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures complied with the Animals (Scientific Procedures) Act 1986 and received university ethical approval of Xiangya Hospital, Central South University (No. 2019030490).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Atesok K, Fu FH, Wolf MR, et al. Augmentation of tendon-to-bone healing. J Bone Joint Surg Am 2014;96:513-21. [Crossref] [PubMed]
- Lu H, Chen C, Xie S, et al. Tendon Healing in Bone Tunnel after Human Anterior Cruciate Ligament Reconstruction: A Systematic Review of Histological Results. J Knee Surg 2019;32:454-62. [Crossref] [PubMed]
- Lu HH, Thomopoulos S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu Rev Biomed Eng 2013;15:201-26. [Crossref] [PubMed]
- Chen C, Zhang T, Liu F, et al. Effect of Low-Intensity Pulsed Ultrasound After Autologous Adipose-Derived Stromal Cell Transplantation for Bone-Tendon Healing in a Rabbit Model. Am J Sports Med 2019;47:942-53. [Crossref] [PubMed]
- Hein J, Reilly JM, Chae J, et al. Retear Rates After Arthroscopic Single-Row, Double-Row, and Suture Bridge Rotator Cuff Repair at a Minimum of 1 Year of Imaging Follow-up: A Systematic Review. Arthroscopy 2015;31:2274-81. [Crossref] [PubMed]
- Depres-Tremblay G, Chevrier A, Snow M, et al. Rotator cuff repair: a review of surgical techniques, animal models, and new technologies under development. J Shoulder Elbow Surg 2016;25:2078-85. [Crossref] [PubMed]
- Klouche S, Lefevre N, Herman S, et al. Return to Sport After Rotator Cuff Tear Repair: A Systematic Review and Meta-analysis. Am J Sports Med 2016;44:1877-87. [Crossref] [PubMed]
- Kuroda R, Usas A, Kubo S, et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum 2006;54:433-42. [Crossref] [PubMed]
- Jang Y, Jung H, Nam Y, et al. Centrifugal gravity-induced BMP4 induces chondrogenic differentiation of adipose-derived stem cells via SOX9 upregulation. Stem Cell Res Ther 2016;7:184. [Crossref] [PubMed]
- Zhang J, Zhu X, Kong Y, et al. Strontium stimulates alkaline phosphatase and bone morphogenetic protein-4 expression in rat chondrocytes cultured in vitro. J Trace Elem Med Biol 2019;55:15-9. [Crossref] [PubMed]
- Miljkovic ND, Cooper GM, Marra KG. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthritis Cartilage 2008;16:1121-30. [Crossref] [PubMed]
- Mifune Y, Matsumoto T, Takayama K, et al. The effect of platelet-rich plasma on the regenerative therapy of muscle derived stem cells for articular cartilage repair. Osteoarthritis Cartilage 2013;21:175-85. [Crossref] [PubMed]
- Diederichs S, Gabler J, Autenrieth J, et al. Differential Regulation of SOX9 Protein During Chondrogenesis of Induced Pluripotent Stem Cells Versus Mesenchymal Stromal Cells: A Shortcoming for Cartilage Formation. Stem Cells Dev 2016;25:598-609. [Crossref] [PubMed]
- Lebaschi AH, Deng XH, Camp CL, et al. Biomechanical, Histologic, and Molecular Evaluation of Tendon Healing in a New Murine Model of Rotator Cuff Repair. Arthroscopy 2018;34:1173-83. [Crossref] [PubMed]
- Wang D, Tan H, Lebaschi AH, et al. Kartogenin Enhances Collagen Organization and Mechanical Strength of the Repaired Enthesis in a Murine Model of Rotator Cuff Repair. Arthroscopy 2018;34:2579-87. [Crossref] [PubMed]
- Ahn SH, Kim CS, Suk HJ, et al. Effect of recombinant human bone morphogenetic protein-4 with carriers in rat calvarial defects. J Periodontol 2003;74:787-97. [Crossref] [PubMed]
- Pang EK, Im SU, Kim CS, et al. Effect of recombinant human bone morphogenetic protein-4 dose on bone formation in a rat calvarial defect model. J Periodontol 2004;75:1364-70. [Crossref] [PubMed]
- Xu D, Zhang T, Qu J, et al. Enhanced patella-patellar tendon healing using combined magnetic fields in a rabbit model. Am J Sports Med 2014;42:2495-501. [Crossref] [PubMed]
- Wada S, Lebaschi AH, Nakagawa Y, et al. Postoperative Tendon Loading With Treadmill Running Delays Tendon-to-Bone Healing: Immunohistochemical Evaluation in a Murine Rotator Cuff Repair Model. J Orthop Res 2019;37:1628-37. [Crossref] [PubMed]
- Dexheimer V, Gabler J, Bomans K, et al. Differential expression of TGF-beta superfamily members and role of Smad1/5/9-signalling in chondral versus endochondral chondrocyte differentiation. Sci Rep 2016;6:36655. [Crossref] [PubMed]
- Ali IH, Brazil DP. Bone morphogenetic proteins and their antagonists: current and emerging clinical uses. Br J Pharmacol 2014;171:3620-32. [Crossref] [PubMed]
- Semba I, Nonaka K, Takahashi I, et al. Positionally-dependent chondrogenesis induced by BMP4 is co-regulated by Sox9 and Msx2. Dev Dyn 2000;217:401-14. [Crossref] [PubMed]
- Nagura I, Kokubu T, Mifune Y, et al. Characterization of progenitor cells derived from torn human rotator cuff tendons by gene expression patterns of chondrogenesis, osteogenesis, and adipogenesis. J Orthop Surg Res 2016;11:40. [Crossref] [PubMed]
- Hu J, Zhang T, Xu D, et al. Combined magnetic fields accelerate bone-tendon junction injury healing through osteogenesis. Scand J Med Sci Sports 2015;25:398-405. [Crossref] [PubMed]
- Lu H, Hu J, Qin L, et al. Area, length and mineralization content of new bone at bone-tendon junction predict its repair quality. J Orthop Res 2011;29:672-7. [Crossref] [PubMed]
- Yu MD, Su BH, Zhang XX. Morphologic and molecular alteration during tibia fracture healing in rat. Eur Rev Med Pharmacol Sci 2018;22:1233-40. [PubMed]
- Lu H, Qin L, Cheung W, et al. Low-intensity pulsed ultrasound accelerated bone-tendon junction healing through regulation of vascular endothelial growth factor expression and cartilage formation. Ultrasound Med Biol 2008;34:1248-60. [Crossref] [PubMed]
- Tang CH, Yang RS, Liou HC, et al. Enhancement of fibronectin synthesis and fibrillogenesis by BMP-4 in cultured rat osteoblast. J Bone Miner Res 2003;18:502-11. [Crossref] [PubMed]
- Zhu W, Kim J, Cheng C, et al. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone 2006;39:61-71. [Crossref] [PubMed]
- Gazzerro E, Gangji V, Canalis E. Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J Clin Invest 1998;102:2106-14. [Crossref] [PubMed]
- Pizette S, Niswander L. BMPs are required at two steps of limb chondrogenesis: formation of prechondrogenic condensations and their differentiation into chondrocytes. Dev Biol 2000;219:237-49. [Crossref] [PubMed]
- Ma CB, Kawamura S, Deng XH, et al. Bone morphogenetic proteins-signaling plays a role in tendon-to-bone healing: a study of rhBMP-2 and noggin. Am J Sports Med 2007;35:597-604. [Crossref] [PubMed]