Chromogranin A as a predictor of radiological disease progression in neuroendocrine tumours
Introduction
Neuroendocrine tumours (NETs) are rare neoplasms, characterised by heterogeneous biological behaviour and clinical course. NETs arise from neuroendocrine cells which are derived from the diffuse endocrine system and represent approximately 2% of all malignant tumours of gastro-entero-pancreatic system (1). NETs could be classified as functioning and non-functioning tumours (2). The recent WHO 2010 classification, which is based on the Ki-67 proliferation index (cells in cycle), divides NETs into three classes with different biological behaviour: low grade NETs (G1), where the proliferation index Ki-67 is ≤2%; intermediate grade NETs (G2), where the Ki-67 index is 3-20% and high-grade neuroendocrine carcinomas (G3), with >20% Ki-67 index (3).
Biochemical markers are evaluated in the blood, urine or other body fluids and are usually elevated in the presence of a tumour (4). In NETs, tumour biomarkers are divided in general, i.e., present in all NETs, and tumour-specific markers (5). In NET management, tumour biomarkers are of critical value, also in view of the obvious limitations of repeated imaging in a disease that is often indolent. Tumour biomarkers are useful for the diagnosis and follow-up of NETs and can be also used as monitor of response to treatment (6). The identification of biomarkers of prognostic value for NETs is urgently needed, to improve patient’s management and tailor therapeutic approach to each single patient.
Chromogranin A (CgA) is found throughout the diffuse neuroendocrine system and is thought to be the best and most sensitive general marker for the diagnosis and follow-up of NETs, with a sensitivity of 96% and 75% in functioning and non-functioning NETs, respectively, and a specificity of 68-100% (7). Immuno-histochemical detection of CgA represents the milestone in the diagnostic work-up of NETs. CgA is a 49 kDa acidic glycoprotein, which represents one of the most abundant components of secretory granules in neuroendocrine cells, and it is secreted from multiple tumours sharing neuroendocrine differentiation (i.e., gastro-entero-pancreatic and bronchial NETs, pheochromocytomas, neuroblastomas, medullary thyroid carcinomas, some pituitary tumours). CgA is considered a sensitive neuroendocrine marker, but its specificity might decrease (up to 68%), as it can be raised also in patients with other malignancies and in different settings, including proton pump inhibitor therapy, chronic atrophic gastritis type A, renal insufficiency, untreated hypertension, liver disease and inflammatory bowel disease (8,9).
Elevated CgA levels have been found in functioning as well as non-functioning tumours, making it a universal marker in NETs (10,11). CgA is more frequently elevated in G1 and G2 tumours compared to G3 NETs (12). The highest CgA levels have been found in metastatic midgut NETs, where CgA appears to correlate with tumour burden and biological activity (12).
CgA has recently been described to be predictive of survival and of treatment response in NETs. CgA has been reported to be useful for the identification of disease progression, and high plasma levels seem to correlate with poor prognosis (13). In a recent study, it was shown that ≥80% reduction in CgA levels following cyto-reductive surgery in gastro-entero-pancreatic NETs is predictive of symptom relief, disease control and improved patient outcomes, even after incomplete cyto-reduction (14). CgA demonstrated clinical value as a prognostic biomarker in retrospective analyses of patients with advanced, well-differentiated NETs (12,14,15). Its levels may correlate with the tumour burden, tumour progression or regression in response to therapy (16). In addition, increased CgA levels seem to be associated with poor progression free survival (PFS) and overall survival (OS) in patients with NETs (11,12,15,16). For instance, the prospective RADIANT-1 study showed that elevated baseline CgA levels were significantly associated with shorter OS in patients with advanced pancreatic NETs (PNETs) (17). According to a recent study, early CgA response seems to be related to therapeutic response in PNET patients, although these findings need to be validated in further prospective, randomised studies (14). A recent prospective Italian study reported that a more than 30%, decrease in CgA levels after acute octreotide administration allows to discriminate those patients responsive to chronic somatostatin analogues (SSAs) treatment from the ones likely unresponsive (18).
In the literature, to our knowledge, there are no available studies which analysed the role of CgA as a predictor of radiological disease progression in all NETs. Therefore, the aim of this study is to evaluate whether CgA increases significantly prior to tumour radiological progression (RP), which would indicate the need of earlier treatment escalation. Furthermore, we also wanted to investigate whether this potential predictive role of CgA is related to any specific subgroup of NETs, although we might be underpowered to perform these analyses.
Materials and methods
Patients with metastatic NETs diagnosed between 1992 and 2011 and evidence of RP, who needed escalation of therapy, were identified from a database at the Neuroendocrine Tumour Unit, Royal Free Hospital, London, UK. Patients had a diagnosis of NET based on morphology and immunohistochemistry and had stage IV disease according to the TNM ENETS criteria (19,20). Metastatic disease was assessed by Radiological Evaluation Criteria in Solid Tumours (RECIST) 1.1 (21).
Clinical data
Clinical data including the time of NET diagnosis, primary tumour site, tumour grade according to WHO 2010 (3), presence and site of metastases, extent of liver involvement (classified as “low extent” if <25%, “moderate extent” if between 25% and 50%, “large extent” if >50%), presence of hormonal syndrome, previous and current treatments were collected for each patient.
Radiological data
All the patients had surveillance scans performed every 3 to 6 months and have had stable disease for at least 12 months before the event of RP. The first event of RP according to RECIST 1.1 criteria was recorded for all patients; moreover, data were also collected for those patients who had two episodes of RP over time, however we had a smaller number of patients for this evaluation. Whole body cross-sectional imaging (computed tomography scan and magnetic resonance imaging), and molecular imaging (i.e., octreotide scan and/or gallium 68 positron emission tomography) studies have been reviewed by Radiologists with great expertise in the NETs, during Multidisciplinary Team Meetings, in order to confirm RP.
Biochemical data
Plasma CgA values at the event of RP and 6 and 12 months prior to this event were retrieved.
CgA was measured using the Imperial Supra-regional Assay Service radioimmunoassay (SAS Hammersmith Hospital, Imperial College, London), which is a competitive radioimmunoassay utilizing polyclonal anti-sera raised against the whole pancreastatin molecule, a 52-amino acid (CgA 250-301) fragment produced by dibasic cleavage of the 439 amino acid CgA peptide; results were expressed as pmol/L. The diagnostic cut-off values for CgA recommended by the manufacturers was <60 pmol/L (22).
All CgA values which were above 1,000 pmol/L or expressed as >1,000 pmol/L were set at 1,000 pmol/L, to avoid discrepancies between the measurements since the exact correspondent CgA value was not available for all the patients.
Statistical analyses
SPSS v21 for Windows (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. CgA was positively skewed which was resistant to transformation, therefore normality assumptions were not met, and non-parametric tests, Wilcox Rank-Sum test (W), were used to evaluate the relationship of different levels of CgA. It is important to mention that there was around 30% of un-systematic missing data on CgA values especially in the older samples. In addition, Spearman Rho correlation was also used to assess the association between variables. Finally, Chi square tests were performed to assess whether there were any important baseline differences between the different type of NETs such as gender and type of NETs.
Results
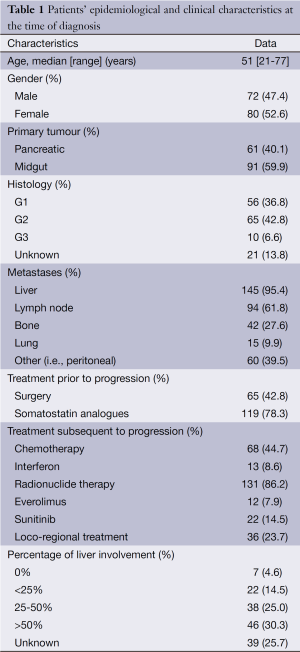
Patients’ epidemiological and clinical characteristics are shown in Table 1.
Full table
A total of 152 patients were assessed including midgut NETs, i.e., NETs involving the jejunum or ileum [91] and PNETs [61], median age 51 years old (range, 21-77 years old). Of these, the grading was as follows: 56 were G1 NETs, 65 G2, 10 G3, 21 of unknown histology. Five PNETs were functioning, including three VIPomas, one gastrinoma and one parathormone related-peptide-secreting PNET. There were no gender differences on the different types of NETS, Chi square showed χ2=0.34, P=0.34.
The majority of the patients (95.4%) had liver metastases, whereas bone and lung metastases were present in a smaller proportion of patients (27.6% and 9.9%, respectively). Among the patients with liver metastases, 30% had more than 50% liver involvement.
The majority of the patients (78%) received SSAs as first-line treatment. In view of advanced and progressive disease, 86% of the patients were treated with radionuclide therapy, 44.7% with chemotherapy, 22% with molecular targeted therapies (i.e., everolimus and/or sunitinib), whilst 8.6% received interferon-A injections. Also liver-directed loco-regional treatments (either hepatic embolization or radiofrequency ablation) were performed in approximately 24% of the patients.
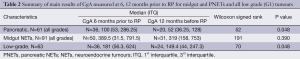
For all NETs, there was a positive trend in terms of increase of CgA values 6 months prior to RP compared to 12 months before RP. Median CgA 6 months before RP was 213 pmol/L [interquartile 1 (Q1) and 3 (Q3) 67 and 664.5 pmol/L, respectively) and at 12 months was 166 pmol/L (Q1 =52 and Q3 =535 pmol/L), W=598.5, P=0.07. Subgroup analysis at first episode of RP showed that for PNETs there was evidence of a difference in the median CgA levels. CgA 6 months before RP was 100 pmol/L (Q1 =53 and Q3 =286.25 pmol/L) and 12 months before was 52 pmol/L (Q1 =36.25 and Q3 =128 pmol/L), W=52, P=0.48. This observation was not confirmed in midgut NETs, where median CgA 6 months before RP was 389.5 pmol/L (Q1 =131.5 and Q3 =791.5 pmol/L) and 12 months before was 319 pmol/L (Q1 =158 and Q3 =753 pmol/L), W=191, P=0.39].
In those NET patients, where data for second episode of RP was available we observed that midgut and PNETs had significantly higher median CgA values at RP than 12 months before [267 (Q1 =66, Q3 =777) vs. 166 (Q1 =52, Q3 =535) pmol/L, T=394.5, P=0.03].
We finally found that low grade tumours (G1) had a median CgA value at 6 months significantly higher than at 12 months [181 (Q1 =56.25, Q3 =624) vs. 149.5 (Q1 =44, Q3 =247.25) pmol/L, W=70, P=0.48].
From the overall sample, 43 patients had a 25% increase of CgA 6 months prior to RP and 31 had a 50% increase 6 months prior to RP.
Table 2 summarises the main results.
Full table
Discussion
This study showed that the increase of CgA levels occurs 6 months prior to RP for PNETs and G1 tumours but not for midgut NETs, according to the subgroup analysis. However, median CgA values at the event of RP were statistically significantly higher when compared to baseline CgA values 12 months before RP. Furthermore, given the overall increase of CgA values during the year preceding RP with a positive trend when comparing CgA values 6 vs. 12 months before RP, we might suggest the inclusion of repeated CgA measurements as a fundamental component of NET follow-up with the aim of identifying those patients who might benefit from a closer follow-up in case of increased CgA values. It is also possible that the study was underpowered to detect some of the differences between the subgroups and therefore larger and prospective studies are needed to confirm the value of CgA as a predictive of RP 6 months prior this occurs.
These findings are of particular relevance, as biomarkers predictive of disease progression are urgently needed, particularly in cases of PNETs, which usually demonstrate a worse prognosis compared to gastrointestinal NETs with a 5-year survival rate of 30-60%, vs. 60-90% for gastrointestinal NETs (23,24). Moreover, the early detection of disease recurrence/progression is of critical value in the therapeutic management of NETs, as surgical therapy is often considered even for metastatic disease, in order to achieve disease control and prolong survival. Moreover, as patients who have had a complete (R0) resection, demonstrate significantly improved outcomes compared to those who have R1 or R2 debulking procedures (25), a detection of disease recurrence/progression at an earlier stage is warranted. This highlights the importance of sensitive tumour biomarkers, as predictors of radiological disease progression.
Our findings are in line with previous studies which highlighted the prognostic value of CgA in patients with advanced well-differentiated NETs (6,12,14,15,26-39). In a recent retrospective study, Welin and co-workers demonstrated that CgA was the first marker to indicate tumour recurrence in the majority of radically operated midgut NETs, suggesting that serial measurements of CgA values should be an essential part in the post-operative workup of those patients. Increasing CgA values during serial measurements might early identify tumour recurrence and might help to assure the best possible treatment for the patients (40). Recently, Yao and co-workers suggested that elevated baseline levels of CgA are of potentially prognostic value (41). In the recent RADIANT-1 study, CgA levels were found to be associated with PFS and OS and an early CgA response (defined as a normalization or ≥30% decrease at week 4 of treatment) was a potential prognostic marker for treatment outcomes in PNET patients treated with everolimus (17,42).
When comparing overall median baseline CgA values 6 and 12 months before RP, we found a positive trend, but the comparison did not reach statistically significance for the whole cohort of patients. The lack of statistical significance could be partially due to the retrospective nature of our study and to the limited sample size. Furthermore, one might speculate that the choice of a different time (i.e., 3 months) before RP to test CgA levels would have been more informative. In fact, we found a positive trend 6 vs. 12 months before the event, but 6 months might be too far from the event of RP to be really predictive. Interestingly, when comparing CgA values 6 vs. 12 months before RP, we found significant results only in the subgroup of PNETs, which might suggest a clinical value of CgA as a predictor of RP mainly in this specific subgroup of patients. Similarly to previous observations (12), we found median CgA to be higher in low and intermediate grade NET rather than in high-grade tumours and we found that CgA shows a predictive value in the subgroup of low-grade NETs.
This is the first study which analysed the role of CgA as a predictor of radiological disease progression in all NETs. A recent study by Jensen et al. reported that an increase of 25% in plasma CgA concentration was useful to indicate tumour progression in a series of 116 patients with ileo-cecal NETs only (43).
The limitations of this study were mainly related to the retrospective nature, the relatively limited sample size and the un-systematic, but relatively large, missing data. Additionally, it would be interesting to test CgA values 3 months before RP, assuming that a closer time might be more informative.
In summary, our results support the clinical utility of CgA as a potential predictor of RP mainly in well-differentiated PNETs and in low-grade (G1) tumours. Further prospective studies with larger number of patients are required prior to conclude that CgA is of prognostic value in predicting RP in NETs and prior to identify specific subgroups of patients (i.e., PNETs) who may benefit from a more intense follow-up, with possible early intervention in case of increased CgA levels.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Warner RR. Enteroendocrine tumors other than carcinoid: a review of clinically significant advances. Gastroenterology 2005;128:1668-84. [PubMed]
- Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61-72. [PubMed]
- Rindi G, Arnold R, Bosman FT, et al. eds. WHO classification of tumors of the digestive system. Lyon: IRAC, 2010:13-14.
- Kilpatrick ES, Lind MJ. Appropriate requesting of serum tumour markers. BMJ 2009;339:b3111. [PubMed]
- Oberg K. Circulating biomarkers in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer 2011;18 Suppl 1:S17-25. [PubMed]
- Duque M, Modlin IM, Gupta A, et al. Biomarkers in neuroendocrine tumors. JOP 2013;14:372-6. [PubMed]
- Baudin E, Bidart JM, Bachelot A, et al. Impact of chromogranin A measurement in the work-up of neuroendocrine tumors. Ann Oncol 2001;12 Suppl 2:S79-82. [PubMed]
- Massironi S, Fraquelli M, Paggi S, et al. Chromogranin A levels in chronic liver disease and hepatocellular carcinoma. Dig Liver Dis 2009;41:31-5. [PubMed]
- Sciola V, Massironi S, Conte D, et al. Plasma chromogranin a in patients with inflammatory bowel disease. Inflamm Bowel Dis 2009;15:867-71. [PubMed]
- Ardill JE, Erikkson B. The importance of the measurement of circulating markers in patients with neuroendocrine tumours of the pancreas and gut. Endocr Relat Cancer 2003;10:459-62. [PubMed]
- Nehar D, Lombard-Bohas C, Olivieri S, et al. Interest of Chromogranin A for diagnosis and follow-up of endocrine tumours. Clin Endocrinol (Oxf) 2004;60:644-52. [PubMed]
- Modlin IM, Gustafsson BI, Moss SF, et al. Chromogranin A--biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol 2010;17:2427-43. [PubMed]
- Rorstad O. Prognostic indicators for carcinoid neuroendocrine tumors of the gastrointestinal tract. J Surg Oncol 2005;89:151-60. [PubMed]
- Jensen EH, Kvols L, McLoughlin JM, et al. Biomarkers predict outcomes following cytoreductive surgery for hepatic metastases from functional carcinoid tumors. Ann Surg Oncol 2007;14:780-5. [PubMed]
- Lawrence B, Gustafsson BI, Kidd M, et al. The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:111-34. [PubMed]
- Korse CM, Taal BG, de Groot CA, et al. Chromogranin-A and N-terminal pro-brain natriuretic peptide: an excellent pair of biomarkers for diagnostics in patients with neuroendocrine tumor. J Clin Oncol 2009;27:4293-9. [PubMed]
- Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol 2008;26:4311-8. [PubMed]
- Massironi S, Conte D, Sciola V, et al. Plasma chromogranin A response to octreotide test: prognostic value for clinical outcome in endocrine digestive tumors. Am J Gastroenterol 2010;105:2072-8. [PubMed]
- Rindi G, Klöppel G, Couvelard A, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2007;451:757-62. [PubMed]
- Rindi G, Klöppel G, Alhman H, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2006;449:395-401. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [PubMed]
- Ramachandran R, Bech P, Murphy KG, et al. Improved diagnostic accuracy for neuroendocrine neoplasms using two chromogranin A assays. Clin Endocrinol (Oxf) 2012;76:831-6. [PubMed]
- Panzuto F, Nasoni S, Falconi M, et al. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer 2005;12:1083-92. [PubMed]
- Tomassetti P, Campana D, Piscitelli L, et al. Endocrine pancreatic tumors: factors correlated with survival. Ann Oncol 2005;16:1806-10. [PubMed]
- Hellman P, Lundström T, Ohrvall U, et al. Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastases. World J Surg 2002;26:991-7. [PubMed]
- Zatelli MC, Torta M, Leon A, et al. Chromogranin A as a marker of neuroendocrine neoplasia: an Italian Multicenter Study. Endocr Relat Cancer 2007;14:473-82. [PubMed]
- Ter-Minassian M, Chan JA, Hooshmand SM, et al. Clinical presentation, recurrence, and survival in patients with neuroendocrine tumors: results from a prospective institutional database. Endocr Relat Cancer 2013;20:187-96. [PubMed]
- Arnold R, Wilke A, Rinke A, et al. Plasma chromogranin A as marker for survival in patients with metastatic endocrine gastroenteropancreatic tumors. Clin Gastroenterol Hepatol 2008;6:820-7. [PubMed]
- Ekeblad S, Skogseid B, Dunder K, et al. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res 2008;14:7798-803. [PubMed]
- van Adrichem RC, Hofland LJ, Feelders RA, et al. Chromogranin A, Ki-67 index and IGF-related genes in patients with neuroendocrine tumors. Endocr Connect 2013;2:172-7. [PubMed]
- Clancy TE, Sengupta TP, Paulus J, et al. Alkaline phosphatase predicts survival in patients with metastatic neuroendocrine tumors. Dig Dis Sci 2006;51:877-84. [PubMed]
- Nykjaer KM, Grønbaek H, Nielsen DT, et al. Description of patients with midgut carcinoid tumours: clinical database from a Danish centre. In Vivo 2007;21:679-84. [PubMed]
- Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 2004;22:4762-71. [PubMed]
- Modlin IM, Moss SF, Oberg K, et al. Gastrointestinal neuroendocrine (carcinoid) tumours: current diagnosis and management. Med J Aust 2010;193:46-52. [PubMed]
- Ahmed A, Turner G, King B, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer 2009;16:885-94. [PubMed]
- Bergestuen DS, Aabakken L, Holm K, et al. Small intestinal neuroendocrine tumors: prognostic factors and survival. Scand J Gastroenterol 2009;44:1084-91. [PubMed]
- Binderup T, Knigge U, Loft A, et al. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res 2010;16:978-85. [PubMed]
- Mateo J, Heymach JV, Zurita AJ. Circulating biomarkers of response to sunitinib in gastroenteropancreatic neuroendocrine tumors: current data and clinical outlook. Mol Diagn Ther 2012;16:151-61. [PubMed]
- Massironi S, Rossi RE, Casazza G, et al. Chromogranin A in diagnosing and monitoring patients with gastroenteropancreatic neuroendocrine neoplasms: a large series from a single institution. Neuroendocrinology 2014;100:240-9. [PubMed]
- Welin S, Stridsberg M, Cunningham J, et al. Elevated plasma chromogranin A is the first indication of recurrence in radically operated midgut carcinoid tumors. Neuroendocrinology 2009;89:302-7. [PubMed]
- Yao JC, Pavel M, Phan AT, et al. Chromogranin A and neuron-specific enolase as prognostic markers in patients with advanced pNET treated with everolimus. J Clin Endocrinol Metab 2011;96:3741-9. [PubMed]
- Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol 2010;28:69-76. [PubMed]
- Jensen KH, Hilsted L, Jensen C, et al. Chromogranin A is a sensitive marker of progression or regression in ileo-cecal neuroendocrine tumors. Scand J Gastroenterol 2013;48:70-7. [PubMed]


